Effects of aging on blood brain barrier and matrix metalloproteases following controlled cortical impact in mice
- PMID: 22201549
- PMCID: PMC4042317
- DOI: 10.1016/j.expneurol.2011.12.016
Effects of aging on blood brain barrier and matrix metalloproteases following controlled cortical impact in mice
Abstract
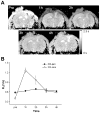
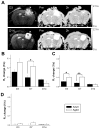
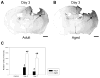
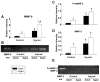
Aging alters the ability of the brain to respond to injury. One of the major differences between the adult and aged brain is that comparable injuries lead to greater blood brain barrier disruption in the aged brain. The goals of these studies were to quantify the effects of age on BBB permeability using high field strength MRI T1 mapping and to determine whether activation of matrix metalloproteases, their inhibitors, or expression of blood brain barrier structural proteins, occludin, zonnula occludins-1 (ZO-1) and claudin-5 were altered following injury to the aged C57/BL6 mouse brain. T1 mapping studies revealed greater blood brain barrier permeability in the aged (21-24 months old) brain than in the adult (4-6 months old) following controlled cortical impact. The increased blood brain barrier permeability in the pericontusional region was confirmed with IgG immunohistochemistry. MMP-9 activity was increased following controlled cortical impact in the aged brain, and this was accompanied by increased MMP-9 gene expression. MMP-2 activity was higher in the uninjured aged brain than in the adult brain. Occludin and ZO-1 mRNA levels were unchanged following injury in either age group, but claudin-5 mRNA levels were lower in the aged than the adult brain following injury. These results demonstrate quantitative increases in blood brain barrier permeability in the aged brain following injury that are accompanied by increased MMP-9 activation and decreased blood brain barrier repair responses.
Copyright © 2011. Published by Elsevier Inc.
Figures
References
-
- Adams DD, Lucas WO, Williams BG, Berkeley BB, Turner KW, Schofield JC. A mouse genetic locus with death clock and life clock features. Mech Ageing Dev. 2001;122:173–89. - PubMed
-
- Berman NE, Marcario JK, Yong C, Raghavan R, Raymond LA, Joag SV, Narayan O, Cheney PD. Microglial activation and neurological symptoms in the SIV model of NeuroAIDS: association of MHC-II and MMP-9 expression with behavioral deficits and evoked potential changes. Neurobiol Dis. 1999;6:486–98. - PubMed
-
- Bilgen M. A new device for experimental modeling of central nervous system injuries. Neurorehabil Neural Repair. 2005;19:219–26. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

