Traumatic brain injury and trichloroethylene exposure interact and produce functional, histological, and mitochondrial deficits
- PMID: 22201550
- PMCID: PMC3294257
- DOI: 10.1016/j.expneurol.2011.12.012
Traumatic brain injury and trichloroethylene exposure interact and produce functional, histological, and mitochondrial deficits
Abstract
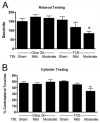
Mitochondria play a pivotal role in the development of pathology associated with Parkinson's disease (PD), traumatic brain injury (TBI), and following exposure to the environmental toxin trichloroethylene (TCE). Evidence from humans indicates that both TBI and TCE can play a role in the development of PD and that each of these insults result in significant mitochondrial dysfunction. In the current studies we hypothesized that exposure to both TCE and TBI would result in increased pathology associated with PD. To test this hypothesis, 16 week old male Fischer 344 rats were administered TCE for either one or two weeks by oral gavage. Following exposure to TCE, rats were subjected to either a sham, mild (1.0mm), or moderate (2.0mm) controlled cortical impact TBI. Given the strong connection between mitochondrial function and PD, TBI, and TCE, tissue from the striatum and substantia nigra were analyzed 6h after the TBI. Neither TCE exposure, TBI, nor the combination of the two insults resulted in mitochondrial deficits at 6h post-TBI in the substantia nigra. Unlike the substantia nigra, the striatum exhibited significant mitochondrial dysfunction. Exposure to TCE alone for two weeks resulted in approximately a 75% reduction in mitochondrial function (p<0.05) in the striatum whereas TBI alone resulted in approximately a 30% reduction in striatal mitochondrial function. Following 1 week exposure to TCE followed by TBI, there was a significant reduction (50%) in mitochondrial function (p<0.05) which required the presence of both insults. Beginning 12 days after the injury significant motor impairment was observed with Rotarod testing. Animals exposed to TCE and a moderate TBI exhibited performance which was approximately 50% of controls (p<0.01). Cylinder testing revealed that at 30 days post-injury animals exposed to TCE and a moderate TBI also had about a 34% reduction in the usage of the contralateral fore paw and this impairment was significantly worse than both control animals and animals exposed to TCE and a mild TBI (p<0.05). At 30 days post-injury there was a 13-17% reduction in the number of tyrosine hydroxylase (TH) positive neurons in the substantia nigra (p<0.05), which was the result of protein loss and not cell death. Loss of TH positive neurons did not result in changes in striatal TH fiber density or levels of the dopamine transporter or type-2 dopamine receptor. Additionally, exposure to TCE prior to the TBI did not increase the loss of cortical tissue, indicating regional specificity for TCE induced deficits. These studies provide further evidence for the connection between TCE, TBI, and PD and lend support to the concept that PD develops from a multifactorial injury scenario.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures
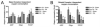




References
-
- Afonso-Oramas D, Cruz-Muros I, Alvarez de la Rosa D, Abreu P, Giraldez T, Castro-Hernandez J, Salas-Hernandez J, Lanciego JL, Rodriguez M, Gonzalez-Hernandez T. (Dopamine transporter glycosylation correlates with the vulnerability of midbrain dopaminergic cells in Parkinson’s disease. Neurobiol Dis. 2009;36:494–508. - PubMed
-
- Akundi RS, Macho A, Munoz E, Lieb K, Bringmann G, Clement HW, Hull M, Fiebich BL. (1-trichloromethyl-1,2,3,4-tetrahydro-beta-carboline-induced apoptosis in the human neuroblastoma cell line SK-N-SH. J Neurochem. 2004;91:263–273. - PubMed
-
- Arundine M, Chopra GK, Wrong A, Lei S, Aarts MM, MacDonald JF, Tymianski M. (Enhanced vulnerability to NMDA toxicity in sublethal traumatic neuronal injury in vitro. J Neurotrauma. 2003;20:1377–1395. - PubMed
-
- ATSDR Toxicological Profile for Trichloroethylene. U.S. Department of Health and Human Services. 1997
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

