Genetic inactivation of IL-1 signaling enhances atherosclerotic plaque instability and reduces outward vessel remodeling in advanced atherosclerosis in mice
- PMID: 22201681
- PMCID: PMC3248279
- DOI: 10.1172/JCI43713
Genetic inactivation of IL-1 signaling enhances atherosclerotic plaque instability and reduces outward vessel remodeling in advanced atherosclerosis in mice
Erratum in
- J Clin Invest. 2012 Feb 1;122(2):783
Abstract
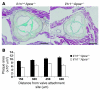
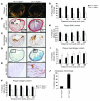
Clinical complications of atherosclerosis arise primarily as a result of luminal obstruction due to atherosclerotic plaque growth, with inadequate outward vessel remodeling and plaque destabilization leading to rupture. IL-1 is a proinflammatory cytokine that promotes atherogenesis in animal models, but its role in plaque destabilization and outward vessel remodeling is unclear. The studies presented herein show that advanced atherosclerotic plaques in mice lacking both IL-1 receptor type I and apolipoprotein E (Il1r1⁻/⁻Apoe⁻/⁻ mice) unexpectedly exhibited multiple features of plaque instability as compared with those of Il1r1⁺/⁺Apoe⁻/⁻ mice. These features included reduced plaque SMC content and coverage, reduced plaque collagen content, and increased intraplaque hemorrhage. In addition, the brachiocephalic arteries of Il1r1⁻/⁻Apoe⁻/⁻ mice exhibited no difference in plaque size, but reduced vessel area and lumen size relative to controls, demonstrating a reduction in outward vessel remodeling. Interestingly, expression of MMP3 was dramatically reduced within the plaque and vessel wall of Il1r1⁻/⁻Apoe⁻/⁻ mice, and Mmp3⁻/⁻Apoe⁻/⁻ mice showed defective outward vessel remodeling compared with controls. In addition, MMP3 was required for IL-1-induced SMC invasion of Matrigel in vitro. Taken together, these results show that IL-1 signaling plays a surprising dual protective role in advanced atherosclerosis by promoting outward vessel remodeling and enhancing features of plaque stability, at least in part through MMP3-dependent mechanisms.
Figures



Comment in
-
IL-1 and atherosclerosis: a murine twist to an evolving human story.J Clin Invest. 2012 Jan;122(1):27-30. doi: 10.1172/JCI61163. Epub 2011 Dec 27. J Clin Invest. 2012. PMID: 22201674 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- T32 GM007267/GM/NIGMS NIH HHS/United States
- R01 HL38854/HL/NHLBI NIH HHS/United States
- 5T32-HL007284/HL/NHLBI NIH HHS/United States
- PG/10/015/28232/BHF_/British Heart Foundation/United Kingdom
- R01 HL057353/HL/NHLBI NIH HHS/United States
- FS/07/053/24069/BHF_/British Heart Foundation/United Kingdom
- R01 HL57353/HL/NHLBI NIH HHS/United States
- R01 HL038854/HL/NHLBI NIH HHS/United States
- P01 HL019242/HL/NHLBI NIH HHS/United States
- 5T32GM007267-26/GM/NIGMS NIH HHS/United States
- T32 HL007284/HL/NHLBI NIH HHS/United States
- P01 HL19242/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

