Substrate channeling in proline metabolism
- PMID: 22201749
- PMCID: PMC3342669
- DOI: 10.2741/3932
Substrate channeling in proline metabolism
Abstract
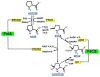
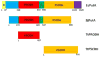
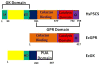
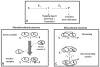
Proline metabolism is an important pathway that has relevance in several cellular functions such as redox balance, apoptosis, and cell survival. Results from different groups have indicated that substrate channeling of proline metabolic intermediates may be a critical mechanism. One intermediate is pyrroline-5-carboxylate (P5C), which upon hydrolysis opens to glutamic semialdehyde (GSA). Recent structural and kinetic evidence indicate substrate channeling of P5C/GSA occurs in the proline catabolic pathway between the proline dehydrogenase and P5C dehydrogenase active sites of bifunctional proline utilization A (PutA). Substrate channeling in PutA is proposed to facilitate the hydrolysis of P5C to GSA which is unfavorable at physiological pH. The second intermediate, gamma-glutamyl phosphate, is part of the proline biosynthetic pathway and is extremely labile. Substrate channeling of gamma-glutamyl phosphate is thought to be necessary to protect it from bulk solvent. Because of the unfavorable equilibrium of P5C/GSA and the reactivity of gamma-glutamyl phosphate, substrate channeling likely improves the efficiency of proline metabolism. Here, we outline general strategies for testing substrate channeling and review the evidence for channeling in proline metabolism.
Figures



References
-
- Donald SP, Sun XY, Hu CA, Yu J, Mei JM, Valle D, Phang JM. Proline oxidase, encoded by p53-induced gene-6, catalyzes the generation of proline-dependent reactive oxygen species. Cancer Res. 2001;61(5):1810–1815. - PubMed
-
- Baumgartner MR, Hu CA, Almashanu S, Steel G, Obie C, Aral B, Rabier D, Kamoun P, Saudubray JM, Valle D. Hyperammonemia with reduced ornithine, citrulline, arginine and proline: a new inborn error caused by a mutation in the gene encoding delta(1)-pyrroline-5-carboxylate synthase. Hum Mol Genet. 2000;9(19):2853–2858. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

