The TR (i)P to Ca²⁺ signaling just got STIMy: an update on STIM1 activated TRPC channels
- PMID: 22201775
- PMCID: PMC3293191
- DOI: 10.2741/3958
The TR (i)P to Ca²⁺ signaling just got STIMy: an update on STIM1 activated TRPC channels
Abstract
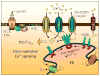
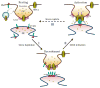
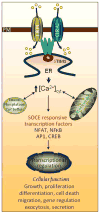
Calcium is a ubiquitous signaling molecule, indispensable for cellular metabolism of organisms from unicellular life forms to higher eukaryotes. The biological function of most eukaryotic cells is uniquely regulated by changes in cytosolic calcium, which is largely achieved by the universal phenomenon of store-operated calcium entry (SOCE). The canonical TRPs and Orai channels have been described as the molecular components of the store-operated calcium channels (SOCC). Importantly, the ER calcium-sensor STIM1 has been shown to initiate SOCE via gating of SOCC. Since the discovery of STIM1, as the critical regulator of SOCE, there has been a flurry of observations suggesting its obligatory role in regulating TRPC and Orai channel function. Considerable effort has been made to identify the molecular details as how STIM1 activates SOCC. In this context, findings as of yet has substantially enriched our understanding on, the modus operandi of SOCE, the distinct cellular locales that organize STIM1-SOCC complexes, and the physiological outcomes entailing STIM1-activated SOCE. In this review we discuss TRPC channels and provide an update on their functional regulation by STIM1.
Figures

References
-
- Berridge MJ. Calcium microdomains: organization and function. Cell Calcium. 2006;40(5–6):405–12. - PubMed
-
- Ambudkar IS. Cellular domains that contribute to Ca2+ entry events. Sci STKE. 2004;2004(243):pe32. - PubMed
-
- Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4(7):517–29. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

