LINEs, SINEs and other retroelements: do birds of a feather flock together?
- PMID: 22201808
- PMCID: PMC3364521
- DOI: 10.2741/3991
LINEs, SINEs and other retroelements: do birds of a feather flock together?
Abstract
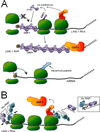
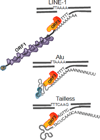
Mobile elements account for almost half of the mass of the human genome. Only the retroelements from the non-LTR (long terminal repeat) retrotransposon family, which include the LINE-1 (L1) and its non-autonomous partners, are currently active and contributing to new insertions. Although these elements seem to share the same basic amplification mechanism, the activity and success of the different types of retroelements varies. For example, Alu-induced mutagenesis is responsible for the majority of the documented instances of human disease induced by insertion of retroelements. Using copy number in mammals as an indicator, some SINEs have been vastly more successful than other retroelements, such as the retropseudogenes and even L1, likely due to differences in post-insertion selection and ability to overcome cellular controls. SINE and LINE integration can be differentially influenced by cellular factors, indicating some differences between in their amplification mechanisms. We focus on the known aspects of this group of retroelements and highlight their similarities and differences that may significantly influence their biological impact.
Figures


Similar articles
-
The RNA polymerase dictates ORF1 requirement and timing of LINE and SINE retrotransposition.PLoS Genet. 2009 Apr;5(4):e1000458. doi: 10.1371/journal.pgen.1000458. Epub 2009 Apr 24. PLoS Genet. 2009. PMID: 19390602 Free PMC article.
-
SINE Retrotransposition: Evaluation of Alu Activity and Recovery of De Novo Inserts.Methods Mol Biol. 2016;1400:183-201. doi: 10.1007/978-1-4939-3372-3_13. Methods Mol Biol. 2016. PMID: 26895055
-
LINEs, SINEs and processed pseudogenes: parasitic strategies for genome modeling.Cytogenet Genome Res. 2005;110(1-4):35-48. doi: 10.1159/000084936. Cytogenet Genome Res. 2005. PMID: 16093656 Review.
-
Recombinant SINEs are formed at high frequency during induced retrotransposition in vivo.Nat Commun. 2012 May 22;3:854. doi: 10.1038/ncomms1855. Nat Commun. 2012. PMID: 22617294
-
[Non-LTR retrotransposons: LINEs and SINEs in plant genome].Yi Chuan. 2006 Jun;28(6):731-6. Yi Chuan. 2006. PMID: 16818439 Review. Chinese.
Cited by
-
Identification of RNA polymerase III-transcribed Alu loci by computational screening of RNA-Seq data.Nucleic Acids Res. 2015 Jan;43(2):817-35. doi: 10.1093/nar/gku1361. Epub 2014 Dec 29. Nucleic Acids Res. 2015. PMID: 25550429 Free PMC article.
-
Alu RNA regulates the cellular pool of active ribosomes by targeted delivery of SRP9/14 to 40S subunits.Nucleic Acids Res. 2015 Mar 11;43(5):2874-87. doi: 10.1093/nar/gkv048. Epub 2015 Feb 19. Nucleic Acids Res. 2015. PMID: 25697503 Free PMC article.
-
Induction of recombination between diverged sequences in a mammalian genome by a double-strand break.Cell Mol Life Sci. 2014 Jun;71(12):2359-71. doi: 10.1007/s00018-013-1520-0. Epub 2013 Nov 21. Cell Mol Life Sci. 2014. PMID: 24257896 Free PMC article.
-
Involvement of Conserved Amino Acids in the C-Terminal Region of LINE-1 ORF2p in Retrotransposition.Genetics. 2017 Mar;205(3):1139-1149. doi: 10.1534/genetics.116.191403. Epub 2017 Jan 18. Genetics. 2017. PMID: 28100588 Free PMC article.
-
A review of bacteria-animal lateral gene transfer may inform our understanding of diseases like cancer.PLoS Genet. 2013;9(10):e1003877. doi: 10.1371/journal.pgen.1003877. Epub 2013 Oct 17. PLoS Genet. 2013. PMID: 24146634 Free PMC article. Review.
References
-
- Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J, Devon K, Dewar K, Doyle M, FitzHugh W, Funke R, Gage D, Harris K, Heaford A, Howland J, Kann L, Lehoczky J, LeVine R, McEwan P, McKernan K, Meldrim J, Mesirov JP, Miranda C, Morris W, Naylor J, Raymond C, Rosetti M, Santos R, Sheridan A, Sougnez C, Stange-Thomann N, Stojanovic N, Subramanian A, Wyman D, Rogers J, Sulston J, Ainscough R, Beck S, Bentley D, Burton J, Clee C, Carter N, Coulson A, Deadman R, Deloukas P, Dunham A, Dunham I, Durbin R, French L, Grafham D, Gregory S, Hubbard T, Humphray S, Hunt A, Jones M, Lloyd C, McMurray A, Matthews L, Mercer S, Milne S, Mullikin JC, Mungall A, Plumb R, Ross M, Shownkeen R, Sims S, Waterston RH, Wilson RK, Hillier LW, McPherson JD, Marra MA, Mardis ER, Fulton LA, Chinwalla AT, Pepin KH, Gish WR, Chissoe SL, Wendl MC, Delehaunty KD, Miner TL, Delehaunty A, Kramer JB, Cook LL, Fulton RS, Johnson DL, Minx PJ, Clifton SW, Hawkins T, Branscomb E, Predki P, Richardson P, Wenning S, Slezak T, Doggett N, Cheng JF, Olsen A, Lucas S, Elkin C, Uberbacher E, Frazier M. Initial sequencing and analysis of the human genome. Nature. 2001;409:860–921. - PubMed
-
- Boeke J, Garfinkel DJ, Styles CA, Fink CR. Ty elements transpose through an RNA intermediate. Cell. 1985;40:491–500. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

