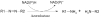Toxicological significance of azo dye metabolism by human intestinal microbiota
- PMID: 22201895
- PMCID: PMC5870118
- DOI: 10.2741/e400
Toxicological significance of azo dye metabolism by human intestinal microbiota
Abstract
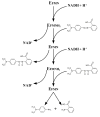
Approximately 0.7 million tons of azo dyes are synthesized each year. Azo dyes are composed of one or more R₁-N=N-R₂ linkages. Studies have shown that both mammalian and microbial azoreductases cleave the azo bonds of the dyes to form compounds that are potentially genotoxic. The human gastrointestinal tract harbors a diverse microbiota comprised of at least several thousand species. Both water-soluble and water-insoluble azo dyes can be reduced by intestinal bacteria. Some of the metabolites produced by intestinal microbiota have been shown to be carcinogenic to humans although the parent azo dyes may not be classified as being carcinogenic. Azoreductase activity is commonly found in intestinal bacteria. Three types of azoreductases have been characterized in bacteria. They are flavin dependent NADH preferred azoreductase, flavin dependent NADPH preferred azoreductase, and flavin free NADPH preferred azoreductase. This review highlights how azo dyes are metabolized by intestinal bacteria, mechanisms of azo reduction, and the potential contribution in the carcinogenesis/mutagenesis of the reduction of the azo dyes by intestinal microbiota.
Figures
Similar articles
-
Reduction of azo dyes and nitroaromatic compounds by bacterial enzymes from the human intestinal tract.Environ Health Perspect. 1995 Jun;103 Suppl 5(Suppl 5):17-9. doi: 10.1289/ehp.95103s417. Environ Health Perspect. 1995. PMID: 8565901 Free PMC article.
-
Metabolism of azo dyes: implication for detoxication and activation.Drug Metab Rev. 1991;23(3-4):253-309. doi: 10.3109/03602539109029761. Drug Metab Rev. 1991. PMID: 1935573 Review.
-
Mutagenicity of azo dyes used in foods, drugs and cosmetics before and after reduction by Clostridium species from the human intestinal tract.Food Chem Toxicol. 1997 Sep;35(9):897-901. doi: 10.1016/s0278-6915(97)00060-4. Food Chem Toxicol. 1997. PMID: 9409630
-
The non-enzymatic reduction of azo dyes by flavin and nicotinamide cofactors under varying conditions.Anaerobe. 2013 Oct;23:87-96. doi: 10.1016/j.anaerobe.2013.07.005. Epub 2013 Jul 26. Anaerobe. 2013. PMID: 23891960
-
Recent advances in azo dye degrading enzyme research.Curr Protein Pept Sci. 2006 Apr;7(2):101-11. doi: 10.2174/138920306776359786. Curr Protein Pept Sci. 2006. PMID: 16611136 Free PMC article. Review.
Cited by
-
Small-Molecule Inhibitors of the CD40-CD40L Costimulatory Protein-Protein Interaction.J Med Chem. 2017 Nov 9;60(21):8906-8922. doi: 10.1021/acs.jmedchem.7b01154. Epub 2017 Oct 25. J Med Chem. 2017. PMID: 29024591 Free PMC article.
-
Effects of Sunset Yellow FCF on Immune System Organs During Different Chicken Embryonic Periods.J Vet Res. 2020 Oct 15;64(4):597-607. doi: 10.2478/jvetres-2020-0064. eCollection 2020 Dec. J Vet Res. 2020. PMID: 33367150 Free PMC article.
-
Toward Small-Molecule Inhibition of Protein-Protein Interactions: General Aspects and Recent Progress in Targeting Costimulatory and Coinhibitory (Immune Checkpoint) Interactions.Curr Top Med Chem. 2018;18(8):674-699. doi: 10.2174/1568026618666180531092503. Curr Top Med Chem. 2018. PMID: 29848279 Free PMC article. Review.
-
Phylogenetically diverse bacteria isolated from tattoo inks, an azo dye-rich environment, decolorize a wide range of azo dyes.Ann Microbiol. 2021 Sep 21;71(1):10.1186/s13213-021-01648-2. doi: 10.1186/s13213-021-01648-2. Ann Microbiol. 2021. PMID: 34744534 Free PMC article.
-
Assessment of synthetic food dye erythrosine induced cytotoxicity, genotoxicity, biochemical and molecular alterations in Allium cepa root meristematic cells: insights from in silico study.Toxicol Res (Camb). 2024 Aug 9;13(4):tfae126. doi: 10.1093/toxres/tfae126. eCollection 2024 Aug. Toxicol Res (Camb). 2024. PMID: 39132191 Free PMC article.
References
-
- Bioassay of p-phenylenediamine dihydrochloride for possible carcinogenicity. Natl Cancer Inst Carcinog Tech Rep Ser. 1979;174:1–107. - PubMed
-
- IARC monographs on the evaluation of the carcinogenic risk of chemicals to man: some aziridines, N-, S- & O-mustards and selenium. IARC Monogr Eval Carcinog Risk Chem Man. 1975;9:1–268. - PubMed
-
- NTP toxicology and carcinogenesis studies of p-nitroaniline (CAS No. 100-01-6) in B6C3F1 mice (gavage studies) Natl Toxicol Program Tech Rep Ser. 1993;418:1–203. - PubMed
-
- Overall evaluations of carcinogenicity: an updating of IARC Monographs volumes 1 to 42. IARC Monogr Eval Carcinog Risks Hum Suppl. 1987;7:1–440. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases