The emerging roles of prohibitins in folliculogenesis
- PMID: 22201905
- PMCID: PMC3267320
- DOI: 10.2741/e410
The emerging roles of prohibitins in folliculogenesis
Abstract
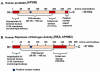
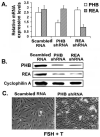
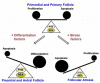
Prohibitins are members of a highly conserved eukaryotic protein family containing the stomatin/prohibitin/flotillin/HflK/C (SPFH) domain (also known as the prohibitin (PHB) domain) found in divergent species from prokaryotes to eukaryotes. Prohibitins are found in unicellular eukaryotes, fungi, plants, animals and humans. Prohibitins are ubiquitously expressed and present in multiple cellular compartments including the mitochondria, nucleus, and the plasma membrane, and shuttles between the mitochondria, cytosol and nucleus. Multiple functions have been attributed to the mitochondrial and nuclear prohibitins, including cellular differentiation, anti-proliferation, and morphogenesis. In the present review, we focus on the recent developments in prohibitins research related to folliculogenesis. Based on current research findings, the data suggest that these molecules play important roles in modulating specific responses of granulose cells to follicle stimulating hormone (FSH) by acting at multiple levels of the FSH signal transduction pathway. Understanding the molecular mechanisms by which the intracellular signaling pathways utilize prohibitins in governing folliculogenesis is likely to result in development of strategies to overcome fertility disorders and suppress ovarian cancer growth.
Figures

Similar articles
-
Prohibitin( PHB) roles in granulosa cell physiology.Cell Tissue Res. 2016 Jan;363(1):19-29. doi: 10.1007/s00441-015-2302-9. Cell Tissue Res. 2016. PMID: 26496733 Free PMC article. Review.
-
Prohibitins role in cellular survival through Ras-Raf-MEK-ERK pathway.J Cell Physiol. 2014 Aug;229(8):998-1004. doi: 10.1002/jcp.24531. J Cell Physiol. 2014. PMID: 24347342 Free PMC article. Review.
-
Mitochondrial targeting of Candida albicans SPFH proteins and requirement of stomatins for SDS-induced stress tolerance.Microbiol Spectr. 2025 Jan 7;13(1):e0173324. doi: 10.1128/spectrum.01733-24. Epub 2024 Dec 6. Microbiol Spectr. 2025. PMID: 39641539 Free PMC article.
-
The Effect of Prohibitins on Mitochondrial Function during Octopus tankahkeei Spermiogenesis.Int J Mol Sci. 2023 Jun 12;24(12):10030. doi: 10.3390/ijms241210030. Int J Mol Sci. 2023. PMID: 37373178 Free PMC article.
-
Prohibitin function within mitochondria: essential roles for cell proliferation and cristae morphogenesis.Biochim Biophys Acta. 2009 Jan;1793(1):27-32. doi: 10.1016/j.bbamcr.2008.05.013. Epub 2008 Jun 17. Biochim Biophys Acta. 2009. PMID: 18558096 Review.
Cited by
-
Mitochondrial dysfunction and adipogenic reduction by prohibitin silencing in 3T3-L1 cells.PLoS One. 2012;7(3):e34315. doi: 10.1371/journal.pone.0034315. Epub 2012 Mar 30. PLoS One. 2012. PMID: 22479600 Free PMC article.
-
Mycobacterial protein PE_PGRS30 induces macrophage apoptosis through prohibitin 2 mitochondrial function interference.Front Microbiol. 2023 Jan 27;14:1080369. doi: 10.3389/fmicb.2023.1080369. eCollection 2023. Front Microbiol. 2023. PMID: 36778852 Free PMC article.
-
Inhibition of the CRAF/prohibitin interaction reverses CRAF-dependent resistance to vemurafenib.Oncogene. 2017 Jan 19;36(3):423-428. doi: 10.1038/onc.2016.214. Epub 2016 Jun 20. Oncogene. 2017. PMID: 27321184
-
hUMSCs restore ovarian function in POI mice by regulating GSK3β-mediated mitochondrial dynamic imbalances in theca cells.Sci Rep. 2024 Aug 16;14(1):19008. doi: 10.1038/s41598-024-69381-9. Sci Rep. 2024. PMID: 39152165 Free PMC article.
-
Association of prohibitin-1 and 2 with oxidative stress in rats with renal interstitial fibrosis.Mol Biol Rep. 2014 May;41(5):3033-43. doi: 10.1007/s11033-014-3162-1. Epub 2014 Mar 5. Mol Biol Rep. 2014. PMID: 24595445
References
-
- Craig J, Orisaka M, Wang H, Orisaka S, Thompson WE, Zhu C, Kotsuji F, Tsang BK. Gonadotropin and intra-ovarian signals regulating follicle development and atresia: the delicate balance between life and death. Front Biosci. 2007;12:3628–3639. - PubMed
-
- Thompson WE, Sanbuissho A, Lee GY, Anderson E. Steroidogenic acute regulatory (StAR) protein (p25) and prohibitin (p28) from cultured rat ovarian granulosa cells. J Reprod Fertil. 1997;109:337–348. - PubMed
-
- Thompson WE, Powell JM, Whittaker JA, Sridaran R, Thomas KH. Immunolocalization and expression of prohibitin, a mitochondrial associated protein within the rat ovaries. Anat Rec. 1999;256:40–48. - PubMed
-
- Thompson WE, Asselin E, Branch A, Stiles JK, Sutovsky P, Lai L, Im G-S, Prather RS, Isom SC, Rucker E, III, Tsang B. Regulation of prohibitin expression during follicular development and atresia in the mammalian ovary. Biol Reprod. 2004;71:282–290. - PubMed
-
- Thompson WE, Branch A, Whittaker JA, Lyn D, Zilberstein M, Mayo KE, Thomas K. Characterization of prohibitin in a newly established rat ovarian granulosa cell line. Endocrinology. 2001;142:4076–4085. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

