Tissue factor: mechanisms of decryption
- PMID: 22201972
- PMCID: PMC3883586
- DOI: 10.2741/477
Tissue factor: mechanisms of decryption
Abstract
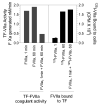
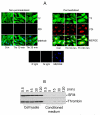
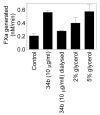
It is generally believed that only a small fraction of the tissue factor (TF) found on cell surfaces is active whereas the vast majority is cryptic in coagulation. It is unclear how cryptic TF differs from the coagulant active TF or potential mechanisms involved in transformation of cryptic TF to the coagulant active form. Exposure of phosphatidylserine (PS) in response to various chemical or pathophysiological stimuli has been considered as the most potent inducer of TF decryption. In addition to PS, TF self-association and association with specialized membrane domains may also play a role in TF decryption. It has been suggested recently that protein disulfide isomerase regulates TF decryption through its oxidoreductase activity by targeting Cys186-Cys209 disulfide bond in TF extracellular domain or regulating the PS equilibrium at the plasma membrane. However, this hypothesis requires further validation to become an accepted mechanism. In this article, we critically review literature on TF encryption/decryption with specific emphasis on recently published data and provide our perspective on this subject.
Figures

Similar articles
-
Tissue factor encryption and decryption: facts and controversies.Thromb Res. 2012 May;129 Suppl 2(Suppl 2):S13-7. doi: 10.1016/j.thromres.2012.02.021. Epub 2012 Mar 6. Thromb Res. 2012. PMID: 22398016 Free PMC article. Review.
-
Encryption and decryption of tissue factor.J Thromb Haemost. 2013 Jun;11 Suppl 1:277-84. doi: 10.1111/jth.12228. J Thromb Haemost. 2013. PMID: 23809131 Review.
-
Contribution of allosteric disulfide in the structural regulation of membrane-bound tissue factor-factor VIIa binary complex.J Biomol Struct Dyn. 2019 Sep;37(14):3707-3720. doi: 10.1080/07391102.2018.1526118. Epub 2018 Nov 13. J Biomol Struct Dyn. 2019. PMID: 30238846
-
Role of PDI in regulating tissue factor: FVIIa activity.Thromb Res. 2010 Apr;125 Suppl 1:S38-41. doi: 10.1016/j.thromres.2010.01.034. Epub 2010 Feb 16. Thromb Res. 2010. PMID: 20163832 Free PMC article. Review.
-
Role of Cell Surface Lipids and Thiol-Disulphide Exchange Pathways in Regulating the Encryption and Decryption of Tissue Factor.Thromb Haemost. 2019 Jun;119(6):860-870. doi: 10.1055/s-0039-1681102. Epub 2019 Mar 12. Thromb Haemost. 2019. PMID: 30861549 Free PMC article. Review.
Cited by
-
Regulation of tissue factor coagulant activity on cell surfaces.J Thromb Haemost. 2012 Nov;10(11):2242-53. doi: 10.1111/jth.12003. J Thromb Haemost. 2012. PMID: 23006890 Free PMC article. Review.
-
Inhibition of phosphoinositol 3 kinase contributes to nanoparticle-mediated exaggeration of endotoxin-induced leukocyte procoagulant activity.Nanomedicine (Lond). 2014 Jul;9(9):1311-26. doi: 10.2217/nnm.13.137. Epub 2013 Nov 27. Nanomedicine (Lond). 2014. PMID: 24279459 Free PMC article.
-
Exercise during hemodialysis does not affect the phenotype or prothrombotic nature of microparticles but alters their proinflammatory function.Physiol Rep. 2018 Sep;6(19):e13825. doi: 10.14814/phy2.13825. Physiol Rep. 2018. PMID: 30294974 Free PMC article. Clinical Trial.
-
Direct Amplification of Tissue Factor:Factor VIIa Procoagulant Activity by Bile Acids Drives Intrahepatic Coagulation.Arterioscler Thromb Vasc Biol. 2019 Oct;39(10):2038-2048. doi: 10.1161/ATVBAHA.119.313215. Epub 2019 Aug 15. Arterioscler Thromb Vasc Biol. 2019. PMID: 31412737 Free PMC article.
-
Platelet P-selectin triggers rapid surface exposure of tissue factor in monocytes.Sci Rep. 2019 Sep 16;9(1):13397. doi: 10.1038/s41598-019-49635-7. Sci Rep. 2019. PMID: 31527604 Free PMC article.
References
-
- Fleck RA, Rao LVM, Rapaport SI, Varki N. Localization of human tissue factor antigen by immunostaining with monospecific, polyclonal anti-human tissue factor antibody. Thromb Res. 1990;59:421–37. - PubMed
-
- Contrino J, Hair G, Kreutzer DL, Rickles FR. In situ detection of tissue factor in vascular endothelial cells: Correlation with the malignant phenotype of human breast disease. Nature Medicine. 1996;2:209–15. - PubMed
-
- Osterud B, Flaegstad T. Increased thromboplastin activity in monocytes of patients with meningococcal infection: Related to an unfavorable prognosis. Thromb Haemost. 1983;49:5–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

