Chimeric antibody receptors (CARs): driving T-cell specificity to enhance anti-tumor immunity
- PMID: 22202074
- PMCID: PMC3889487
- DOI: 10.2741/s282
Chimeric antibody receptors (CARs): driving T-cell specificity to enhance anti-tumor immunity
Abstract
Adoptive transfer of antigen-specific T cells is a compelling tool to treat cancer. To overcome issues of immune tolerance which limits the endogenous adaptive immune response to tumor-associated antigens, robust systems for the genetic modification and characterization of T cells expressing chimeric antigen receptors (CARs) to redirect specificity have been produced. Refinements with regards to persistence and trafficking of the genetically modified T cells are underway to help improve the potency of genetically modified T cells. Clinical trials utilizing this technology demonstrate feasibility, and increasingly, antitumor activity, paving the way for multi-center trials to establish the efficacy of this novel T-cell therapy.
Figures
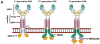
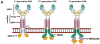
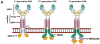
References
-
- Cullis JO, Jiang YZ, Schwarer AP, Hughes TP, Barrett AJ, Goldman JM. Donor leukocyte infusions for chronic myeloid leukemia in relapse after allogeneic bone marrow transplantation. Blood. 1992;79(5):1379–81. - PubMed
-
- Drobyski WR, Keever CA, Roth MS, Koethe S, Hanson G, McFadden P, Gottschall JL, Ash RC, van Tuinen P, Horowitz MM, et al. Salvage immunotherapy using donor leukocyte infusions as treatment for relapsed chronic myelogenous leukemia after allogeneic bone marrow transplantation: efficacy and toxicity of a defined T-cell dose. Blood. 1993;82(8):2310–8. - PubMed
-
- Ferster A, Bujan W, Mouraux T, Devalck C, Heimann P, Sariban E. Complete remission following donor leukocyte infusion in ALL relapsing after haploidentical bone marrow transplantation. Bone Marrow Transplant. 1994;14(2):331–2. - PubMed
-
- Kolb HJ, Mittermuller J, Clemm C, Holler E, Ledderose G, Brehm G, Heim M, Wilmanns W. Donor leukocyte transfusions for treatment of recurrent chronic myelogenous leukemia in marrow transplant patients. Blood. 1990;76(12):2462–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
