The role of mTOR signaling in Alzheimer disease
- PMID: 22202101
- PMCID: PMC4111148
- DOI: 10.2741/s310
The role of mTOR signaling in Alzheimer disease
Abstract
The buildup of Abeta and tau is believed to directly cause or contribute to the progressive cognitive deficits characteristic of Alzheimer disease. However, the molecular pathways linking Abeta and tau accumulation to learning and memory deficits remain elusive. There is growing evidence that soluble forms of Abeta and tau can obstruct learning and memory by interfering with several signaling cascades. In this review, I will present data showing that the mammalian target of rapamycin (mTOR) may play a role in Abeta and tau induced neurodegeneration.
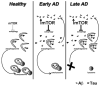
Figures
Similar articles
-
Molecular interplay between mammalian target of rapamycin (mTOR), amyloid-beta, and Tau: effects on cognitive impairments.J Biol Chem. 2010 Apr 23;285(17):13107-20. doi: 10.1074/jbc.M110.100420. Epub 2010 Feb 23. J Biol Chem. 2010. PMID: 20178983 Free PMC article.
-
Mammalian target of rapamycin: a valid therapeutic target through the autophagy pathway for Alzheimer's disease?J Neurosci Res. 2012 Jun;90(6):1105-18. doi: 10.1002/jnr.23011. Epub 2012 Feb 16. J Neurosci Res. 2012. PMID: 22344941 Review.
-
Naturally secreted amyloid-beta increases mammalian target of rapamycin (mTOR) activity via a PRAS40-mediated mechanism.J Biol Chem. 2011 Mar 18;286(11):8924-32. doi: 10.1074/jbc.M110.180638. Epub 2011 Jan 25. J Biol Chem. 2011. PMID: 21266573 Free PMC article.
-
Rapamycin inhibits activation of AMPK-mTOR signaling pathway-induced Alzheimer's disease lesion in hippocampus of rats with type 2 diabetes mellitus.Int J Neurosci. 2019 Feb;129(2):179-188. doi: 10.1080/00207454.2018.1491571. Epub 2018 Nov 5. Int J Neurosci. 2019. PMID: 29962282
-
mTOR in Down syndrome: Role in Aß and tau neuropathology and transition to Alzheimer disease-like dementia.Free Radic Biol Med. 2018 Jan;114:94-101. doi: 10.1016/j.freeradbiomed.2017.08.009. Epub 2017 Aug 12. Free Radic Biol Med. 2018. PMID: 28807816 Free PMC article. Review.
Cited by
-
MicroRNA-Target Interaction Regulatory Network in Alzheimer's Disease.J Pers Med. 2021 Dec 2;11(12):1275. doi: 10.3390/jpm11121275. J Pers Med. 2021. PMID: 34945753 Free PMC article.
-
Milk's Role as an Epigenetic Regulator in Health and Disease.Diseases. 2017 Mar 15;5(1):12. doi: 10.3390/diseases5010012. Diseases. 2017. PMID: 28933365 Free PMC article. Review.
-
The Brain-Gut Axis, an Important Player in Alzheimer and Parkinson Disease: A Narrative Review.J Clin Med. 2024 Jul 15;13(14):4130. doi: 10.3390/jcm13144130. J Clin Med. 2024. PMID: 39064171 Free PMC article. Review.
-
DL0410, a novel dual cholinesterase inhibitor, protects mouse brains against Aβ-induced neuronal damage via the Akt/JNK signaling pathway.Acta Pharmacol Sin. 2016 Nov;37(11):1401-1412. doi: 10.1038/aps.2016.87. Epub 2016 Aug 8. Acta Pharmacol Sin. 2016. PMID: 27498773 Free PMC article.
-
Development of Dementia in Type 2 Diabetes Patients: Mechanisms of Insulin Resistance and Antidiabetic Drug Development.Cells. 2022 Nov 25;11(23):3767. doi: 10.3390/cells11233767. Cells. 2022. PMID: 36497027 Free PMC article. Review.
References
-
- 2010 Alzheimer's disease facts and figures. Alzheimers Dement. 2010;6:158–94. - PubMed
-
- Hirtz D, Thurman DJ, Gwinn-Hardy K, Mohamed M, Chaudhuri AR, Zalutsky R. How common are the “common” neurologic disorders? Neurology. 2007;68:326–37. - PubMed
-
- Querfurth HW, LaFerla FM. Alzheimer's disease. N Engl J Med. 2010;362:329–44. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

