Late intervention with anti-BRAF(V600E) therapy induces tumor regression in an orthotopic mouse model of human anaplastic thyroid cancer
- PMID: 22202162
- PMCID: PMC3275388
- DOI: 10.1210/en.2011-1519
Late intervention with anti-BRAF(V600E) therapy induces tumor regression in an orthotopic mouse model of human anaplastic thyroid cancer
Abstract
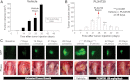
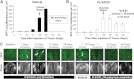
Human anaplastic thyroid cancer (ATC) is a lethal disease with an advanced clinical presentation and median survival of 3 months. The BRAF(V600E) oncoprotein is a potent transforming factor that causes human thyroid cancer cell progression in vitro and in vivo; therefore, we sought to target this oncoprotein in a late intervention model of ATC in vivo. We used the human ATC cell line 8505c, which harbors the BRAF(V600E) and TP53(R248G) mutations. Immunocompromised mice were randomized to receive the selective anti-BRAF(V600E) inhibitor, PLX4720, or vehicle by oral gavage 28 d after tumor implantation, 1 wk before all animals typically die due to widespread metastatic lung disease and neck compressive symptoms in this model. Mice were euthanized weekly to evaluate tumor volume and metastases. Control mice showed progressive tumor growth and lung metastases by 35 d after tumor implantation. At that time, all control mice had large tumors, were cachectic, and were euthanized due to their tumor-related weight loss. PLX4720-treated mice, however, showed a significant decrease in tumor volume and lung metastases in addition to a reversal of tumor-related weight loss. Mouse survival was extended to 49 d in PLX4720-treated animals. PLX4720 treatment inhibited cell cycle progression from 28 d to 49 d in vivo. PLX4720 induces striking tumor regression and reversal of cachexia in an in vivo model of advanced thyroid cancer that harbors the BRAF(V600E) mutation.
Figures





Similar articles
-
Targeting BRAFV600E with PLX4720 displays potent antimigratory and anti-invasive activity in preclinical models of human thyroid cancer.Oncologist. 2011;16(3):296-309. doi: 10.1634/theoncologist.2010-0317. Epub 2011 Feb 25. Oncologist. 2011. PMID: 21355020 Free PMC article.
-
Combined BRAF(V600E)- and SRC-inhibition induces apoptosis, evokes an immune response and reduces tumor growth in an immunocompetent orthotopic mouse model of anaplastic thyroid cancer.Oncotarget. 2014 Jun 30;5(12):3996-4010. doi: 10.18632/oncotarget.2130. Oncotarget. 2014. PMID: 24994118 Free PMC article.
-
Thyroidectomy with neoadjuvant PLX4720 extends survival and decreases tumor burden in an orthotopic mouse model of anaplastic thyroid cancer.Surgery. 2010 Dec;148(6):1154-62; discussion 1162. doi: 10.1016/j.surg.2010.09.001. Surgery. 2010. PMID: 21134546 Free PMC article.
-
Orthotopic mouse models for the preclinical and translational study of targeted therapies against metastatic human thyroid carcinoma with BRAF(V600E) or wild-type BRAF.Oncogene. 2014 Nov 20;33(47):5397-404. doi: 10.1038/onc.2013.544. Epub 2013 Dec 23. Oncogene. 2014. PMID: 24362526 Free PMC article. Review.
-
BRAF as a target for cancer therapy.Anticancer Agents Med Chem. 2011 Mar;11(3):285-95. doi: 10.2174/187152011795347469. Anticancer Agents Med Chem. 2011. PMID: 21426297 Review.
Cited by
-
Clinical application of molecular testing of fine-needle aspiration specimens in thyroid nodules.Otolaryngol Clin North Am. 2014 Aug;47(4):557-71. doi: 10.1016/j.otc.2014.04.003. Epub 2014 Jun 12. Otolaryngol Clin North Am. 2014. PMID: 25041958 Free PMC article. Review.
-
The role of chemotherapy and latest emerging target therapies in anaplastic thyroid cancer.Onco Targets Ther. 2013 Sep 16;9:1231-41. doi: 10.2147/OTT.S46545. Onco Targets Ther. 2013. PMID: 24092989 Free PMC article. Review.
-
Preclinical Imaging for the Study of Mouse Models of Thyroid Cancer.Int J Mol Sci. 2017 Dec 16;18(12):2731. doi: 10.3390/ijms18122731. Int J Mol Sci. 2017. PMID: 29258188 Free PMC article. Review.
-
Expression of angiogenic switch, cachexia and inflammation factors at the crossroad in undifferentiated thyroid carcinoma with BRAF(V600E).Cancer Lett. 2016 Oct 1;380(2):577-585. doi: 10.1016/j.canlet.2015.07.012. Epub 2015 Jul 17. Cancer Lett. 2016. PMID: 26189429 Free PMC article.
-
Targeting PEAK1 sensitizes anaplastic thyroid carcinoma cells harboring BRAFV600E to Vemurafenib by Bim upregulation.Histol Histopathol. 2024 Sep;39(9):1159-1165. doi: 10.14670/HH-18-705. Epub 2024 Jan 8. Histol Histopathol. 2024. PMID: 38284248
References
-
- Neff RL, Farrar WB, Kloos RT, Burman KD. 2008. Anaplastic thyroid cancer. Endocrinol Metab Clin North Am 37:525–538 - PubMed
-
- Are C, Shaha AR. 2006. Anaplastic thyroid carcinoma: biology, pathogenesis, prognostic factors, and treatment approaches. Ann Surg Oncol 13:453–464 - PubMed
-
- Broome JT, Gauger PG, Miller BS, Doherty GM. 2009. Anaplastic thyroid cancer manifesting as new-onset Horner syndrome. Endocr Pract 15:563–566 - PubMed
-
- Pasieka JL. 2003. Anaplastic thyroid cancer. Curr Opin Oncol 15:78–83 - PubMed
-
- Braga-Basaria M, Ringel MD. 2003. Clinical review 158: Beyond radioiodine: a review of potential new therapeutic approaches for thyroid cancer. J Clin Endocrinol Metab 88:1947–1960 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

