Sexually dimorphic testosterone secretion in prenatal and neonatal mice is independent of kisspeptin-Kiss1r and GnRH signaling
- PMID: 22202164
- PMCID: PMC3275395
- DOI: 10.1210/en.2011-1838
Sexually dimorphic testosterone secretion in prenatal and neonatal mice is independent of kisspeptin-Kiss1r and GnRH signaling
Abstract
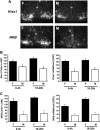
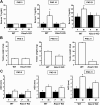
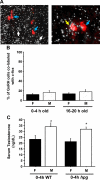
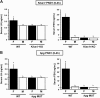
Kisspeptin, encoded by the Kiss1 gene, stimulates GnRH secretion and is therefore critical for sex steroid secretion at puberty and in adulthood. However, kisspeptin's role in regulating sex steroid secretion earlier in development is unexplored. In rodents, testosterone (T) levels are higher in prenatal and newborn males than females. We determined whether kisspeptin-Kiss1r and GnRH signaling plays a role in sexually dimorphic perinatal T secretion in mice. Our results demonstrate that 1) T levels in newborn males are elevated at 4 h but not 20 h after birth, but hypothalamic Kiss1 and neurokinin B (NKB) levels in males are not different between these time points (and both are lower than in females); 2) serum T levels in newborn Kiss1r knockout (KO) males are higher than in newborn females and similar to wild-type (WT) males; 3) perinatal hypothalamic progesterone receptor (Pgr) expression, which is dependent on circulating levels of gonadally produced T, is significantly higher in prenatal and newborn Kiss1r KO and WT males than similarly aged females; 4) multiple measures of testicular growth and function are not different between developing Kiss1r KO and WT mice until after postnatal d 5; and 5) GnRH neurons of newborn males do not exhibit high c-fos coexpression, and newborn hypogonadal (hpg) male mice (lacking GnRH) secrete elevated T, similar to newborn WT males. We conclude that, unlike in puberty and adulthood, elevated T secretion in prenatal and neonatal mice is independent of both kisspeptin and GnRH signaling, and the necessity of kisspeptin-Kiss1r signaling for testicular function is first apparent after d 5.
Figures




Similar articles
-
Analysis of multiple positive feedback paradigms demonstrates a complete absence of LH surges and GnRH activation in mice lacking kisspeptin signaling.Biol Reprod. 2013 Jun 13;88(6):146. doi: 10.1095/biolreprod.113.108555. Print 2013 Jun. Biol Reprod. 2013. PMID: 23595904 Free PMC article.
-
The kisspeptin receptor GPR54 is required for sexual differentiation of the brain and behavior.J Neurosci. 2007 Aug 15;27(33):8826-35. doi: 10.1523/JNEUROSCI.2099-07.2007. J Neurosci. 2007. PMID: 17699664 Free PMC article.
-
Impaired kisspeptin signaling decreases metabolism and promotes glucose intolerance and obesity.J Clin Invest. 2014 Jul;124(7):3075-9. doi: 10.1172/JCI71075. Epub 2014 Jun 17. J Clin Invest. 2014. PMID: 24937427 Free PMC article.
-
Role of Kisspeptin and NKB in Puberty in Nonhuman Primates: Sex Differences.Semin Reprod Med. 2019 Mar;37(2):47-55. doi: 10.1055/s-0039-3400253. Epub 2019 Dec 17. Semin Reprod Med. 2019. PMID: 31847024 Free PMC article. Review.
-
[Kisspeptin and leptin in the regulation of fertility].Mol Biol (Mosk). 2015 Sep-Oct;49(5):707-15. doi: 10.7868/S0026898415050134. Mol Biol (Mosk). 2015. PMID: 26510589 Review. Russian.
Cited by
-
Neuroendocrine control of the onset of puberty.Front Neuroendocrinol. 2015 Jul;38:73-88. doi: 10.1016/j.yfrne.2015.04.002. Epub 2015 Apr 22. Front Neuroendocrinol. 2015. PMID: 25913220 Free PMC article. Review.
-
Lactation undernutrition leads to multigenerational molecular programming of hypothalamic gene networks controlling reproduction.BMC Genomics. 2016 May 4;17:333. doi: 10.1186/s12864-016-2615-4. BMC Genomics. 2016. PMID: 27146259 Free PMC article.
-
Microarray analysis of perinatal-estrogen-induced changes in gene expression related to brain sexual differentiation in mice.PLoS One. 2013 Nov 4;8(11):e79437. doi: 10.1371/journal.pone.0079437. eCollection 2013. PLoS One. 2013. PMID: 24223949 Free PMC article.
-
The GnRH Antagonist Degarelix Suppresses Gonadotropin Secretion and Pituitary Sensitivity in Midgestation Sheep Fetuses.Endocrinology. 2022 Feb 1;163(2):bqab262. doi: 10.1210/endocr/bqab262. Endocrinology. 2022. PMID: 34958103 Free PMC article.
-
Daily successive changes in reproductive gene expression and neuronal activation in the brains of pubertal female mice.Mol Cell Endocrinol. 2015 Feb 5;401:84-97. doi: 10.1016/j.mce.2014.11.025. Epub 2014 Dec 8. Mol Cell Endocrinol. 2015. PMID: 25498961 Free PMC article.
References
-
- Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O'Rahilly S, Carlton MB, Crowley WF, Jr, Aparicio SA, Colledge WH. 2003. The GPR54 gene as a regulator of puberty. N Engl J Med 349:1614–1627 - PubMed
-
- Messager S, Chatzidaki EE, Ma D, Hendrick AG, Zahn D, Dixon J, Thresher RR, Malinge I, Lomet D, Carlton MB, Colledge WH, Caraty A, Aparicio SA. 2005. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci USA 102:1761–1766 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

