Fibronectin extra domain-A promotes hepatic stellate cell motility but not differentiation into myofibroblasts
- PMID: 22202457
- PMCID: PMC3321084
- DOI: 10.1053/j.gastro.2011.12.038
Fibronectin extra domain-A promotes hepatic stellate cell motility but not differentiation into myofibroblasts
Abstract
Background & aims: Myofibroblasts are the primary cell type involved in physiologic wound healing and its pathologic counterpart, fibrosis. Cellular fibronectin that contains the alternatively spliced extra domain A (EIIIA) is up-regulated during these processes and is believed to promote myofibroblast differentiation. We sought to determine the requirement for EIIIA in fibrosis and differentiation of myofibroblasts in rodent livers.
Methods: We used a mechanically tunable hydrogel cell culture system to study differentiation of primary hepatic stellate cells and portal fibroblasts from rats into myofibroblasts. Liver fibrosis was induced in mice by bile duct ligation or administration of thioacetamide.
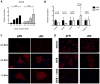
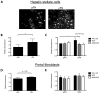
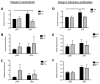
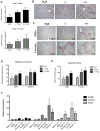
Results: EIIIA was not required for differentiation of rat hepatic stellate cells or portal fibroblasts into fibrogenic myofibroblasts. Instead, hepatic stellate cells cultured on EIIIA-containing cellular fibronectin formed increased numbers of lamellipodia; their random motility and chemotaxis also increased. These increases required the receptor for EIIIA, the integrin α(9)β(1). In contrast, the motility of portal fibroblasts did not increase on EIIIA, and these cells expressed little α(9)β(1). Male EIIIA(-/-) mice were modestly protected from thioacetamide-induced fibrosis, which requires motile hepatic stellate cells, but not from bile duct ligation-induced fibrosis, in which portal fibroblasts are more important. Notably, myofibroblasts developed during induction of fibrosis with either thioacetamide or bile duct ligation in EIIIA(-/-) mice.
Conclusions: EIIIA is dispensable for differentiation of hepatic stellate cells and portal fibroblasts to myofibroblasts, both in culture and in mouse models of fibrosis. Our findings, however, indicate a role for EIIIA in promoting stellate cell motility and parenchymal liver fibrosis.
Copyright © 2012 AGA Institute. Published by Elsevier Inc. All rights reserved.
Figures


Similar articles
-
The myofibroblastic conversion of peribiliary fibrogenic cells distinct from hepatic stellate cells is stimulated by platelet-derived growth factor during liver fibrogenesis.Lab Invest. 2003 Feb;83(2):163-73. doi: 10.1097/01.lab.0000054178.01162.e4. Lab Invest. 2003. PMID: 12594232
-
Fibromodulin, an oxidative stress-sensitive proteoglycan, regulates the fibrogenic response to liver injury in mice.Gastroenterology. 2012 Mar;142(3):612-621.e5. doi: 10.1053/j.gastro.2011.11.029. Epub 2011 Dec 1. Gastroenterology. 2012. PMID: 22138190 Free PMC article.
-
Expression of variant fibronectins in wound healing: cellular source and biological activity of the EIIIA segment in rat hepatic fibrogenesis.J Cell Biol. 1994 Dec;127(6 Pt 2):2037-48. doi: 10.1083/jcb.127.6.2037. J Cell Biol. 1994. PMID: 7806580 Free PMC article.
-
The characteristics of activated portal fibroblasts/myofibroblasts in liver fibrosis.Differentiation. 2016 Sep;92(3):84-92. doi: 10.1016/j.diff.2016.07.001. Epub 2016 Aug 31. Differentiation. 2016. PMID: 27591095 Free PMC article. Review.
-
Peribiliary myofibroblasts in biliary type liver fibrosis.Front Biosci. 2002 Feb 1;7:d496-503. doi: 10.2741/A790. Front Biosci. 2002. PMID: 11815289 Review.
Cited by
-
Cellular and molecular mechanisms in the pathogenesis of liver fibrosis: An update.World J Gastroenterol. 2014 Jun 21;20(23):7260-76. doi: 10.3748/wjg.v20.i23.7260. World J Gastroenterol. 2014. PMID: 24966597 Free PMC article. Review.
-
Biology of portal hypertension.Hepatol Int. 2018 Feb;12(Suppl 1):11-23. doi: 10.1007/s12072-017-9826-x. Epub 2017 Oct 26. Hepatol Int. 2018. PMID: 29075990 Free PMC article. Review.
-
Molecular Mechanism Responsible for Fibronectin-controlled Alterations in Matrix Stiffness in Advanced Chronic Liver Fibrogenesis.J Biol Chem. 2016 Jan 1;291(1):72-88. doi: 10.1074/jbc.M115.691519. Epub 2015 Nov 9. J Biol Chem. 2016. PMID: 26553870 Free PMC article.
-
Dynamics of compartment-specific proteomic landscapes of hepatotoxic and cholestatic models of liver fibrosis.Elife. 2025 Apr 8;13:RP98023. doi: 10.7554/eLife.98023. Elife. 2025. PMID: 40197391 Free PMC article.
-
The fibronectin ED-A domain enhances recruitment of latent TGF-β-binding protein-1 to the fibroblast matrix.J Cell Sci. 2018 Mar 1;131(5):jcs201293. doi: 10.1242/jcs.201293. J Cell Sci. 2018. PMID: 29361522 Free PMC article.
References
-
- Schwarzbauer JE, Tamkun JW, Lemischka IR, Hynes RO. Three different fibronectin mRNAs arise by alternative splicing within the coding region. Cell. 1983;35:421–31. - PubMed
-
- Peters JH, Hynes RO. Fibronectin isoform distribution in the mouse. I. The alternatively spliced EIIIB, EIIIA, and V segments show widespread codistribution in the developing mouse embryo. Cell Adhes Commun. 1996;4:103–25. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

