Assessment of prognostic circulating tumor cells in a phase III trial of adjuvant immunotherapy after complete resection of stage IV melanoma
- PMID: 22202581
- PMCID: PMC3320770
- DOI: 10.1097/SLA.0b013e3182380f56
Assessment of prognostic circulating tumor cells in a phase III trial of adjuvant immunotherapy after complete resection of stage IV melanoma
Abstract
Objective: To verify circulating tumor cell (CTC) prognostic utility in stage IV resected melanoma patients in a prospective international phase III clinical trial.
Background: Our studies of melanoma patients in phase II clinical trials demonstrated prognostic significance for CTCs in patients with AJCC stage IV melanoma. CTCs were assessed to determine prognostic utility in follow-up of disease-free stage IV patients pre- and during treatment.
Methods: After complete metastasectomy, patients were prospectively enrolled in a randomized trial of adjuvant therapy with a whole-cell melanoma vaccine, Canvaxin, plus Bacille Calmette-Guerin (BCG) versus placebo plus BCG. Blood specimens obtained pretreatment (n = 244) and during treatment (n = 214) were evaluated by quantitative real-time reverse-transcriptase polymerase chain reaction (qPCR) for expression of MART-1, MAGE-A3, and PAX3 mRNA biomarkers. Univariate and multivariate Cox analyses examined CTC biomarker expression with respect to clinicopathological variables.
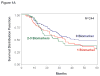
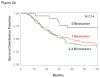
Results: CTC biomarker(s) (≥ 1) was detected in 54% of patients pretreatment and in 86% of patients over the first 3 months. With a median follow-up of 21.9 months, 71% of patients recurred and 48% expired. CTC levels were not associated with known prognostic factors or treatment arm. In multivariate analysis, pretreatment CTC (> 0 vs. 0 biomarker) status was significantly associated with disease-free survival (DFS; HR 1.64, P = 0.002) and overall survival (OS; HR 1.53, P = 0.028). Serial CTC (>0 vs. 0 biomarker) status was also significantly associated with DFS (HR 1.91, P = 0.02) and OS (HR 2.57, P = 0.012).
Conclusion: CTC assessment can provide prognostic discrimination before and during adjuvant treatment for resected stage IV melanoma patients.
Trial registration: ClinicalTrials.gov NCT00052156.
Figures


References
-
- Barth A, Wanek LA, Morton DL. Prognostic factors in 1,521 melanoma patients with distant metastases. J Am Coll Surg. 1995;181(3):193–201. - PubMed
-
- Ollila DW. Complete metastasectomy in patients with stage IV metastatic melanoma. Lancet Oncol. 2006;7(11):919–24. - PubMed
-
- Ollila DW, Stern SL, Morton DL. Tumor doubling time: a selection factor for pulmonary resection of metastatic melanoma. J Surg Oncol. 1998;69(4):206–11. - PubMed
-
- Mocellin S, Del Fiore P, Guarnieri L, et al. Molecular detection of circulating tumor cells is an independent prognostic factor in patients with high-risk cutaneous melanoma. Int J Cancer. 2004;111(5):741–5. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

