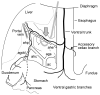
Vagal innervation of the hepatic portal vein and liver is not necessary for Roux-en-Y gastric bypass surgery-induced hypophagia, weight loss, and hypermetabolism
- PMID: 22202582
- PMCID: PMC3259289
- DOI: 10.1097/SLA.0b013e31823e71b7
Vagal innervation of the hepatic portal vein and liver is not necessary for Roux-en-Y gastric bypass surgery-induced hypophagia, weight loss, and hypermetabolism
Abstract
Objective: To determine the role of the common hepatic branch of the abdominal vagus on the beneficial effects of Roux-en-Y gastric bypass (RYGB) on weight loss, food intake, food choice, and energy expenditure in a rat model.
Background: Although changes in gut hormone patterns are the leading candidates in RYGB's effects on appetite, weight loss, and reversal of diabetes, a potential role for afferent signaling through the vagal hepatic branch potentially sensing glucose levels in the hepatic portal vein has recently been suggested in a mouse model of RYGB.
Methods: Male Sprague-Dawley rats underwent either RYGB alone (RYGB; n = 7), RYGB + common hepatic branch vagotomy (RYGB + HV; n = 6), or sham procedure (sham; n = 9). Body weight, body composition, meal patterns, food choice, energy expenditure, and fecal energy loss were monitored up to 3 months after intervention.
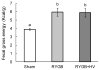
Results: Both RYGB and RYGB + HV significantly reduced body weight, adiposity, meal size, and fat preference, and increased satiety, energy expenditure, and respiratory exchange rate compared with sham procedure, and there were no significant differences in these effects between RYGB and RYGB + HV rats.
Conclusions: Integrity of vagal nerve supply to the liver, hepatic portal vein, and the proximal duodenum provided by the common hepatic branch is not necessary for RYGB to reduce food intake and body weight or increase energy expenditure. Specifically, it is unlikely that a hepatic portal vein glucose sensor signaling RYGB-induced increased intestinal gluconeogenesis to the brain depends on vagal afferent fibers.
Figures
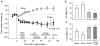

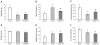


Similar articles
-
Vagal innervation of intestine contributes to weight loss After Roux-en-Y gastric bypass surgery in rats.Obes Surg. 2014 Dec;24(12):2145-51. doi: 10.1007/s11695-014-1338-3. Obes Surg. 2014. PMID: 24972684 Free PMC article.
-
Impact of the hepatic branch of the vagus and Roux-en-Y gastric bypass on the hypoglycemic effect and glucagon-like peptide-1 in rats with type 2 diabetes mellitus.J Surg Res. 2014 Sep;191(1):123-9. doi: 10.1016/j.jss.2014.03.062. Epub 2014 Mar 26. J Surg Res. 2014. PMID: 24768143
-
Does the hepatic branch of vagus mediate the secretion of glucagon-like peptide-1 during the Roux-en-Y gastric bypass surgery?J Gastrointest Surg. 2014 Nov;18(11):1957-64. doi: 10.1007/s11605-014-2632-z. Epub 2014 Sep 3. J Gastrointest Surg. 2014. Retraction in: J Gastrointest Surg. 2016 Jan;20(1):230. doi: 10.1007/s11605-015-3020-z. PMID: 25183408 Retracted.
-
Mechanisms of improved glycaemic control after Roux-en-Y gastric bypass.Dan Med J. 2015 Apr;62(4):B5057. Dan Med J. 2015. PMID: 25872541 Review.
-
What is the Mechanism Behind Weight Loss Maintenance with Gastric Bypass?Curr Obes Rep. 2015 Jun;4(2):262-8. doi: 10.1007/s13679-015-0158-7. Curr Obes Rep. 2015. PMID: 26627220 Review.
Cited by
-
The physiology underlying Roux-en-Y gastric bypass: a status report.Am J Physiol Regul Integr Comp Physiol. 2014 Dec 1;307(11):R1275-91. doi: 10.1152/ajpregu.00185.2014. Epub 2014 Sep 24. Am J Physiol Regul Integr Comp Physiol. 2014. PMID: 25253084 Free PMC article. Review.
-
Regulation of body weight: Lessons learned from bariatric surgery.Mol Metab. 2023 Feb;68:101517. doi: 10.1016/j.molmet.2022.101517. Epub 2022 May 26. Mol Metab. 2023. PMID: 35644477 Free PMC article. Review.
-
Gastrointestinal hormones and bariatric surgery-induced weight loss.Obesity (Silver Spring). 2013 Jun;21(6):1093-103. doi: 10.1002/oby.20364. Obesity (Silver Spring). 2013. PMID: 23512841 Free PMC article. Review.
-
Glucose metabolism after bariatric surgery: implications for T2DM remission and hypoglycaemia.Nat Rev Endocrinol. 2023 Mar;19(3):164-176. doi: 10.1038/s41574-022-00757-5. Epub 2022 Oct 26. Nat Rev Endocrinol. 2023. PMID: 36289368 Free PMC article. Review.
-
Does gastric bypass surgery change body weight set point?Int J Obes Suppl. 2016 Dec;6(Suppl 1):S37-S43. doi: 10.1038/ijosup.2016.9. Epub 2016 Nov 16. Int J Obes Suppl. 2016. PMID: 28685029 Free PMC article. Review.
References
-
- Cummings DE. Endocrine mechanisms mediating remission of diabetes after gastric bypass surgery. Int J Obes (Lond) 2009;33 (Suppl 1):S33–40. - PubMed
-
- le Roux CW, Welbourn R, Werling M, et al. Gut hormones as mediators of appetite and weight loss after Roux-en-Y gastric bypass. Ann Surg. 2007;246(5):780–5. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

