Trends in the susceptibility of clinically important resistant bacteria to tigecycline: results from the Tigecycline In Vitro Surveillance in Taiwan study, 2006 to 2010
- PMID: 22203598
- PMCID: PMC3294947
- DOI: 10.1128/AAC.06053-11
Trends in the susceptibility of clinically important resistant bacteria to tigecycline: results from the Tigecycline In Vitro Surveillance in Taiwan study, 2006 to 2010
Abstract
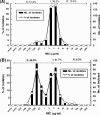
The Tigecycline In Vitro Surveillance in Taiwan (TIST) study, a nationwide, prospective surveillance during 2006 to 2010, collected a total of 7,793 clinical isolates, including methicillin-resistant Staphylococcus aureus (MRSA) (n = 1,834), penicillin-resistant Streptococcus pneumoniae (PRSP) (n = 423), vancomycin-resistant enterococci (VRE) (n = 219), extended-spectrum β-lactamase (ESBL)-producing Escherichia coli (n = 1,141), ESBL-producing Klebsiella pneumoniae (n = 1,330), Acinetobacter baumannii (n = 1,645), and Stenotrophomonas maltophilia (n = 903), from different specimens from 20 different hospitals in Taiwan. MICs of tigecycline were determined following the criteria of the U.S. Food and Drug Administration (FDA) and the European Committee on Antimicrobial Susceptibility Testing (EUCAST-2011). Among drug-resistant Gram-positive pathogens, all of the PRSP isolates were susceptible to tigecycline (MIC(90), 0.03 μg/ml), and only one MRSA isolate (MIC(90), 0.5 μg/ml) and three VRE isolates (MIC(90), 0.125 μg/ml) were nonsusceptible to tigecycline. Among the Gram-negative bacteria, the tigecycline susceptibility rates were 99.65% for ESBL-producing E. coli (MIC(90), 0.5 μg/ml) and 96.32% for ESBL-producing K. pneumoniae (MIC(90), 2 μg/ml) when interpreted by FDA criteria but were 98.7% and 85.8%, respectively, when interpreted by EUCAST-2011 criteria. The susceptibility rate for A. baumannii (MIC(90), 4 μg/ml) decreased from 80.9% in 2006 to 55.3% in 2009 but increased to 73.4% in 2010. A bimodal MIC distribution was found among carbapenem-susceptible A. baumannii isolates, and a unimodal MIC distribution was found among carbapenem-nonsusceptible A. baumannii isolates. In Taiwan, tigecycline continues to have excellent in vitro activity against several major clinically important drug-resistant bacteria, with the exception of A. baumannii.
Figures


References
-
- Boucher HW, et al. 2009. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin. Infect. Dis. 48:1–12 - PubMed
-
- Bouchillon SK, Iredell JR, Barkham T, Lee K, Dowzicky MJ. 2009. Comparative in vitro activity of tigecycline and other antimicrobials against Gram-negative and Gram-positive organisms collected from the Asia-Pacific Rim as part of the Tigecycline Evaluation and Surveillance Trial (TEST). Int. J. Antimicrob. Agents 33:130–136 - PubMed
-
- Chen YH, et al. 2011. Antimicrobial susceptibility profiles of aerobic and facultative Gram-negative bacilli isolated from patients with intra-abdominal infections in the Asia-Pacific region according to currently established susceptibility interpretive criteria. J. Infect. 62:280–291 - PubMed
-
- Chen WY, Jang TN, Huang CH, Hsueh PR. 2009. In vitro susceptibilities of aerobic and facultative anaerobic Gram-negative bacilli isolated from patients with intra-abdominal infections at a medical center in Taiwan: results of the Study for Monitoring Antimicrobial Resistance Trends (SMART) 2002-2006. J. Microbiol. Immunol. Infect. 42:317–323 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

