Phylogeny and ontogeny of the habenular structure
- PMID: 22203792
- PMCID: PMC3244072
- DOI: 10.3389/fnins.2011.00138
Phylogeny and ontogeny of the habenular structure
Abstract
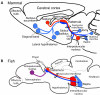
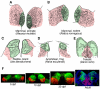
Habenula is an epithalamic nucleus connecting the forebrain with the ventral midbrain and hindbrain that plays a pivotal role in decision making by regulating dopaminergic and serotonergic activities. Intriguingly, habenula has also attracted interest as a model for brain asymmetry, since many vertebrates show left-right differences in habenula size and neural circuitry. Despite the functional significance of this nucleus, few studies have addressed the molecular mechanisms underlying habenular development. Mammalian habenula consists of the medial and lateral habenulae, which have distinct neural connectivity. The habenula shows phylogenetic conservation from fish to human, and studies using genetically accessible model animals have provided molecular insights into the developmental mechanisms of the habenula. The results suggest that development of the habenular asymmetry is mediated by differential regulation of the neurogenetic period for generating specific neuronal subtypes. Since the orientation and size ratio of the medial and lateral habenulae differ across species, the evolution of those subregions within the habenula may also reflect changes in neurogenesis duration for each habenular subdivision according to the evolutionary process.
Keywords: brain asymmetry; evolution; habenula; interpeduncular nucleus; lateralization; monoamines; neurogenesis; zebrafish.
Figures

References
-
- Agetsuma M., Aizawa H., Aoki T., Nakayama R., Takahoko M., Goto M., Sassa T., Amo R., Shiraki T., Kawakami K., Hosoya T., Higashijima S., Okamoto H. (2010). The habenula is crucial for experience-dependent modification of fear responses in zebrafish. Nat. Neurosci. 13, 1354–1356 10.1038/nn.2654 - DOI - PubMed
-
- Aizawa H., Bianco I. H., Hamaoka T., Miyashita T., Uemura O., Concha M. L., Russell C., Wilson S. W., Okamoto H. (2005). Laterotopic representation of left-right information onto the dorso-ventral axis of a zebrafish midbrain target nucleus. Curr. Biol. 15, 238–243 10.1016/j.cub.2005.01.014 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

