Prey capture behavior evoked by simple visual stimuli in larval zebrafish
- PMID: 22203793
- PMCID: PMC3240898
- DOI: 10.3389/fnsys.2011.00101
Prey capture behavior evoked by simple visual stimuli in larval zebrafish
Abstract
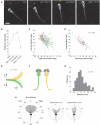
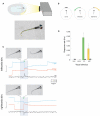
Understanding how the nervous system recognizes salient stimuli in the environment and selects and executes the appropriate behavioral responses is a fundamental question in systems neuroscience. To facilitate the neuroethological study of visually guided behavior in larval zebrafish, we developed "virtual reality" assays in which precisely controlled visual cues can be presented to larvae whilst their behavior is automatically monitored using machine vision algorithms. Freely swimming larvae responded to moving stimuli in a size-dependent manner: they directed multiple low amplitude orienting turns (∼20°) toward small moving spots (1°) but reacted to larger spots (10°) with high-amplitude aversive turns (∼60°). The tracking of small spots led us to examine how larvae respond to prey during hunting routines. By analyzing movie sequences of larvae hunting paramecia, we discovered that all prey capture routines commence with eye convergence and larvae maintain their eyes in a highly converged position for the duration of the prey-tracking and capture swim phases. We adapted our virtual reality assay to deliver artificial visual cues to partially restrained larvae and found that small moving spots evoked convergent eye movements and J-turns of the tail, which are defining features of natural hunting. We propose that eye convergence represents the engagement of a predatory mode of behavior in larval fish and serves to increase the region of binocular visual space to enable stereoscopic targeting of prey.
Keywords: behavior; binocular vision; ocular vergence; prey capture; zebrafish.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

