Sperm-borne microRNA-34c is required for the first cleavage division in mouse
- PMID: 22203953
- PMCID: PMC3258645
- DOI: 10.1073/pnas.1110368109
Sperm-borne microRNA-34c is required for the first cleavage division in mouse
Abstract
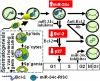
In mammals, the sperm deliver mRNA of unknown function into the oocytes during fertilization. The role of sperm microRNAs (miRNAs) in preimplantation development is unknown. miRNA profiling identified six miRNAs expressed in the sperm and the zygotes but not in the oocytes or preimplantation embryos. Sperm contained both the precursor and the mature form of one of these miRNAs, miR-34c. The absence of an increased level of miR-34c in zygotes derived from α-amanitin-treated oocytes and in parthenogenetic oocytes supported a sperm origin of zygotic miR-34c. Injection of miR-34c inhibitor into zygotes inhibited DNA synthesis and significantly suppressed first cleavage division. A 3' UTR luciferase assay and Western blotting demonstrated that miR-34c regulates B-cell leukemia/lymphoma 2 (Bcl-2) expression in the zygotes. Coinjection of anti-Bcl-2 antibody in zygotes partially reversed but injection of Bcl-2 protein mimicked the effect of miR-34c inhibition. Oocyte activation is essential for the miR-34c action in zygotes, as demonstrated by a decrease in 3'UTR luciferase reporter activity and Bcl-2 expression after injection of precursor miR-34c into parthenogenetic oocytes. Our findings provide evidence that sperm-borne miR-34c is important for the first cell division via modulation of Bcl-2 expression.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
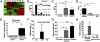


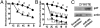

References
-
- Krawetz SA. Paternal contribution: New insights and future challenges. Nat Rev Genet. 2005;6:633–642. - PubMed
-
- Wu AT, et al. PAWP, a sperm-specific WW domain-binding protein, promotes meiotic resumption and pronuclear development during fertilization. J Biol Chem. 2007;282:12164–12175. - PubMed
-
- Dadoune JP. Spermatozoal RNAs: What about their functions? Microsc Res Tech. 2009;72:536–551. - PubMed
-
- Ostermeier GC, Miller D, Huntriss JD, Diamond MP, Krawetz SA. Reproductive biology: Delivering spermatozoan RNA to the oocyte. Nature. 2004;429:154. - PubMed
-
- Rassoulzadegan M, et al. RNA-mediated non-Mendelian inheritance of an epigenetic change in the mouse. Nature. 2006;441:469–474. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

