Vertebrate-like regeneration in the invertebrate chordate amphioxus
- PMID: 22203957
- PMCID: PMC3258630
- DOI: 10.1073/pnas.1100045109
Vertebrate-like regeneration in the invertebrate chordate amphioxus
Abstract
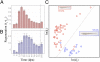
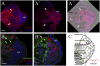
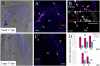
An important question in biology is why some animals are able to regenerate, whereas others are not. The basal chordate amphioxus is uniquely positioned to address the evolution of regeneration. We report here the high regeneration potential of the European amphioxus Branchiostoma lanceolatum. Adults regenerate both anterior and posterior structures, including neural tube, notochord, fin, and muscle. Development of a classifier based on tail regeneration profiles predicts the assignment of young and old adults to their own class with >94% accuracy. The process involves loss of differentiated characteristics, formation of an msx-expressing blastema, and neurogenesis. Moreover, regeneration is linked to the activation of satellite-like Pax3/7 progenitor cells, the extent of which declines with size and age. Our results provide a framework for understanding the evolution and diversity of regeneration mechanisms in vertebrates.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
AmphiPax3/7, an amphioxus paired box gene: insights into chordate myogenesis, neurogenesis, and the possible evolutionary precursor of definitive vertebrate neural crest.Evol Dev. 1999 Nov-Dec;1(3):153-65. doi: 10.1046/j.1525-142x.1999.99019.x. Evol Dev. 1999. PMID: 11324100
-
Amphioxus makes the cut-Again.Commun Integr Biol. 2012 Sep 1;5(5):499-502. doi: 10.4161/cib.21075. Commun Integr Biol. 2012. PMID: 23739582 Free PMC article.
-
Insights into spawning behavior and development of the European amphioxus (Branchiostoma lanceolatum).J Exp Zool B Mol Dev Evol. 2007 Jul 15;308(4):484-93. doi: 10.1002/jez.b.21179. J Exp Zool B Mol Dev Evol. 2007. PMID: 17520703
-
Amphioxus regeneration: evolutionary and biomedical implications.Int J Dev Biol. 2017;61(10-11-12):689-696. doi: 10.1387/ijdb.170219is. Int J Dev Biol. 2017. PMID: 29319117 Review.
-
The basal chordate amphioxus as a simple model for elucidating developmental mechanisms in vertebrates.Birth Defects Res C Embryo Today. 2008 Sep;84(3):175-87. doi: 10.1002/bdrc.20128. Birth Defects Res C Embryo Today. 2008. PMID: 18773463 Review.
Cited by
-
Three amphioxus reference genomes reveal gene and chromosome evolution of chordates.Proc Natl Acad Sci U S A. 2023 Mar 7;120(10):e2201504120. doi: 10.1073/pnas.2201504120. Epub 2023 Mar 3. Proc Natl Acad Sci U S A. 2023. PMID: 36867684 Free PMC article.
-
Exposure to elevated sea-surface temperatures below the bleaching threshold impairs coral recovery and regeneration following injury.PeerJ. 2017 Aug 18;5:e3719. doi: 10.7717/peerj.3719. eCollection 2017. PeerJ. 2017. PMID: 28828283 Free PMC article.
-
Beyond Adult Stem Cells: Dedifferentiation as a Unifying Mechanism Underlying Regeneration in Invertebrate Deuterostomes.Front Cell Dev Biol. 2020 Oct 20;8:587320. doi: 10.3389/fcell.2020.587320. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 33195242 Free PMC article. Review.
-
All for One and One for All: Regenerating Skeletal Muscle.Cold Spring Harb Perspect Biol. 2022 Aug 1;14(8):a040824. doi: 10.1101/cshperspect.a040824. Cold Spring Harb Perspect Biol. 2022. PMID: 34750174 Free PMC article. Review.
-
The salamander blastema within the broader context of metazoan regeneration.Front Cell Dev Biol. 2023 Aug 11;11:1206157. doi: 10.3389/fcell.2023.1206157. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37635872 Free PMC article. Review.
References
-
- Bely AE, Nyberg KG. Evolution of animal regeneration: Re-emergence of a field. Trends Ecol Evol. 2010;25:161–170. - PubMed
-
- Tanaka EM, Ferretti P. Considering the evolution of regeneration in the central nervous system. Nat Rev Neurosci. 2009;10:713–723. - PubMed
-
- Sánchez Alvarado A, Tsonis PA. Bridging the regeneration gap: Genetic insights from diverse animal models. Nat Rev Genet. 2006;7:873–884. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

