Disordered form of the scaffold protein IscU is the substrate for iron-sulfur cluster assembly on cysteine desulfurase
- PMID: 22203963
- PMCID: PMC3258623
- DOI: 10.1073/pnas.1114372109
Disordered form of the scaffold protein IscU is the substrate for iron-sulfur cluster assembly on cysteine desulfurase
Abstract
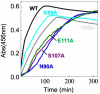
The scaffold protein for iron-sulfur cluster assembly, apo-IscU, populates two interconverting conformational states, one disordered (D) and one structured (S) as revealed by extensive NMR assignments. At pH 8 and 25 °C, approximately 70% of the protein is S, and the lifetimes of the states are 1.3 s (S) and 0.50 s (D). Zn(II) and Fe(II) each bind and stabilize structured (S-like) states. Single amino acid substitutions at conserved residues were found that shift the equilibrium toward either the S or the D state. Cluster assembly takes place in the complex between IscU and the cysteine desulfurase, IscS, and our NMR studies demonstrate that IscS binds preferentially the D form of apo-IscU. The addition of 10% IscS to IscU was found to greatly increase H/D exchange at protected amides of IscU, to increase the rate of the S → D reaction, and to decrease the rate of the D → S reaction. In the saturated IscU:IscS complex, IscU is largely disordered. In vitro cluster assembly reactions provided evidence for the functional importance of the S&lrarr2;D equilibrium. IscU variants that favor the S state were found to undergo a lag phase, not observed with the wild type, that delayed cluster assembly; variants that favor the D state were found to assemble less stable clusters at an intermediate rate without the lag. It appears that IscU has evolved to exist in a disordered conformational state that is the initial substrate for the desulfurase and to convert to a structured state that stabilizes the cluster once it is assembled.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


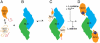
Similar articles
-
Human mitochondrial chaperone (mtHSP70) and cysteine desulfurase (NFS1) bind preferentially to the disordered conformation, whereas co-chaperone (HSC20) binds to the structured conformation of the iron-sulfur cluster scaffold protein (ISCU).J Biol Chem. 2013 Oct 4;288(40):28755-70. doi: 10.1074/jbc.M113.482042. Epub 2013 Aug 12. J Biol Chem. 2013. PMID: 23940031 Free PMC article.
-
The scaffold protein IscU retains a structured conformation in the Fe-S cluster assembly complex.Chembiochem. 2014 Jul 21;15(11):1682-6. doi: 10.1002/cbic.201402211. Epub 2014 Jul 8. Chembiochem. 2014. PMID: 25044349
-
Metamorphic protein IscU alternates conformations in the course of its role as the scaffold protein for iron-sulfur cluster biosynthesis and delivery.FEBS Lett. 2013 Apr 17;587(8):1172-9. doi: 10.1016/j.febslet.2013.01.003. Epub 2013 Jan 16. FEBS Lett. 2013. PMID: 23333622 Free PMC article. Review.
-
ISCU(M108I) and ISCU(D39V) Differ from Wild-Type ISCU in Their Failure To Form Cysteine Desulfurase Complexes Containing Both Frataxin and Ferredoxin.Biochemistry. 2018 Mar 6;57(9):1491-1500. doi: 10.1021/acs.biochem.7b01234. Epub 2018 Feb 14. Biochemistry. 2018. PMID: 29406711 Free PMC article.
-
Molecular chaperones HscA/Ssq1 and HscB/Jac1 and their roles in iron-sulfur protein maturation.Crit Rev Biochem Mol Biol. 2007 Mar-Apr;42(2):95-111. doi: 10.1080/10409230701322298. Crit Rev Biochem Mol Biol. 2007. PMID: 17453917 Review.
Cited by
-
Analysis of Reconstituted Tripartite Complex Supports Avidity-based Recruitment of Hsp70 by Substrate Bound J-domain Protein.J Mol Biol. 2023 Nov 1;435(21):168283. doi: 10.1016/j.jmb.2023.168283. Epub 2023 Sep 18. J Mol Biol. 2023. PMID: 37730084 Free PMC article.
-
The conformational landscape of fold-switcher KaiB is tuned to the circadian rhythm timescale.Proc Natl Acad Sci U S A. 2024 Nov 5;121(45):e2412293121. doi: 10.1073/pnas.2412293121. Epub 2024 Oct 30. Proc Natl Acad Sci U S A. 2024. PMID: 39475637 Free PMC article.
-
NMR as a Tool to Investigate the Processes of Mitochondrial and Cytosolic Iron-Sulfur Cluster Biosynthesis.Molecules. 2018 Aug 31;23(9):2213. doi: 10.3390/molecules23092213. Molecules. 2018. PMID: 30200358 Free PMC article. Review.
-
Fe-S cluster biogenesis in Gram-positive bacteria: SufU is a zinc-dependent sulfur transfer protein.Biochemistry. 2014 Jan 14;53(1):152-60. doi: 10.1021/bi4011978. Epub 2013 Dec 23. Biochemistry. 2014. PMID: 24321018 Free PMC article.
-
Human mitochondrial chaperone (mtHSP70) and cysteine desulfurase (NFS1) bind preferentially to the disordered conformation, whereas co-chaperone (HSC20) binds to the structured conformation of the iron-sulfur cluster scaffold protein (ISCU).J Biol Chem. 2013 Oct 4;288(40):28755-70. doi: 10.1074/jbc.M113.482042. Epub 2013 Aug 12. J Biol Chem. 2013. PMID: 23940031 Free PMC article.
References
-
- Johnson DC, Dean DR, Smith AD, Johnson MK. Structure, function, and formation of biological iron-sulfur clusters. Annu Rev Biochem. 2005;74:247–281. - PubMed
-
- Sheftel A, Stehling O, Lill R. Iron-sulfur proteins in health and disease. Trends Endocrinol Metab. 2010;21:302–314. - PubMed
-
- Vickery LE, Cupp-Vickery JR. Molecular chaperones HscA/Ssq1 and HscB/Jac1 and their roles in iron-sulfur protein maturation. Crit Rev Biochem Mol Biol. 2007;42:95–111. - PubMed
-
- Cupp-Vickery JR, Urbina H, Vickery LE. Crystal structure of IscS, a cysteine desulfurase from Escherichia coli. J Mol Biol. 2003;330:1049–1059. - PubMed
-
- Urbina HD, Silberg JJ, Hoff KG, Vickery LE. Transfer of sulfur from IscS to IscU during Fe/S cluster assembly. J Biol Chem. 2001;276:44521–44526. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

