Alpha-1-antitrypsin monotherapy reduces graft-versus-host disease after experimental allogeneic bone marrow transplantation
- PMID: 22203983
- PMCID: PMC3258603
- DOI: 10.1073/pnas.1117665109
Alpha-1-antitrypsin monotherapy reduces graft-versus-host disease after experimental allogeneic bone marrow transplantation
Abstract
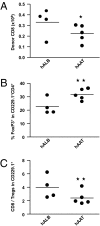
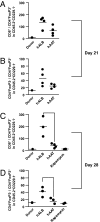
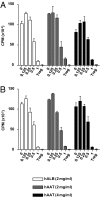
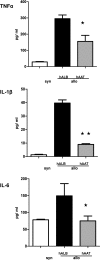
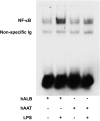
Acute graft-versus-host disease (GvHD) is a major complication that prevents successful outcomes after allogeneic bone marrow transplantation (BMT), an effective therapy for hematological malignancies. Several studies demonstrate that donor T cells and host antigen-presenting cells along with several proinflammatory cytokines are required for the induction of GvHD and contribute to its severity. Increasing evidence demonstrates that human serum-derived αalpha-1- anti-trypsin (AAT) reduces production of proinflammatory cytokines, induces anti-inflammatory cytokines, and interferes with maturation of dendritic cells. Using well-characterized mouse models of BMT, we have studied the effects of AAT on GvHD severity. Administration of AAT early after BMT decreased mortality in three models of GvHD and reduced serum levels of proinflammatory cytokines in the allogeneic recipients compared with vehicle (albumin) treated animals. AAT treatment reduced the expansion of alloreactive T effector cells but enhanced the recovery of T regulatory T cells, (Tregs) thus altering the ratio of donor T effector to T regulatory cells in favor of reducing the pathological process. However, despite altering the ratio in vivo, AAT had no direct effects on either the donor T effector cells or T regulatory cells Tregs in vitro. In contrast, AAT suppressed LPS-induced in vitro secretion of proinflammatory cytokines such as TNF-α and IL-1β, enhanced the production of the anti-inflammatory cytokine IL-10, and impaired NF-κB translocation in the host dendritic cells. In light of its long history of safety in humans, these findings suggest that administration of AAT represents a novel unique and viable strategy to mitigate clinical GvHD.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Appelbaum FR. Hematopoietic-cell transplantation at 50. N Engl J Med. 2007;357:1472–1475. - PubMed
-
- Welniak LA, Blazar BR, Murphy WJ. Immunobiology of allogeneic hematopoietic stem cell transplantation. Annu Rev Immunol. 2007;25:139–170. - PubMed
-
- Antin JH, Ferrara JL. Cytokine dysregulation and acute graft-versus-host disease. Blood. 1992;80:2964–2968. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

