AMP-activated protein kinase is physiologically regulated by inositol polyphosphate multikinase
- PMID: 22203993
- PMCID: PMC3258619
- DOI: 10.1073/pnas.1119751109
AMP-activated protein kinase is physiologically regulated by inositol polyphosphate multikinase
Abstract
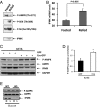
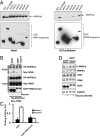
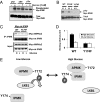
The AMP-activated kinase (AMPK) senses the energy status of cells and regulates fuel availability, whereas hypothalamic AMPK regulates food intake. We report that inositol polyphosphate multikinase (IPMK) regulates glucose signaling to AMPK in a pathway whereby glucose activates phosphorylation of IPMK at tyrosine 174 enabling the enzyme to bind to AMPK and regulate its activation. Thus, refeeding fasted mice rapidly and markedly stimulates transcriptional enhancement of IPMK expression while down-regulating AMPK. Also, AMPK is up-regulated in mice with genetic depletion of hypothalamic IPMK. IPMK physiologically binds AMPK, with binding enhanced by glucose treatment. Regulation by glucose of phospho-AMPK in hypothalamic cell lines is prevented by blocking AMPK-IPMK binding. These findings imply that IPMK inhibitors will be beneficial in treating obesity and diabetes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Carling D. The AMP-activated protein kinase cascade—a unifying system for energy control. Trends Biochem Sci. 2004;29:18–24. - PubMed
-
- Hardie DG, Pan DA. Regulation of fatty acid synthesis and oxidation by the AMP-activated protein kinase. Biochem Soc Trans. 2002;30:1064–1070. - PubMed
-
- Minokoshi Y, et al. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature. 2004;428:569–574. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

