The channel kinase, TRPM7, is required for early embryonic development
- PMID: 22203997
- PMCID: PMC3277139
- DOI: 10.1073/pnas.1120033109
The channel kinase, TRPM7, is required for early embryonic development
Abstract
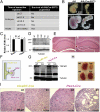
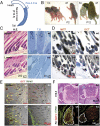
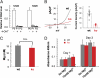
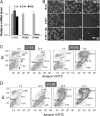
Global disruption of transient receptor potential-melastatin-like 7 (Trpm7) in mice results in embryonic lethality before embryonic day 7. Using tamoxifen-inducible disruption of Trpm7 and multiple Cre recombinase lines, we show that Trpm7 deletion before and during organogenesis results in severe tissue-specific developmental defects. We find that Trpm7 is essential for kidney development from metanephric mesenchyme but not ureteric bud. Disruption of neural crest Trpm7 at early stages results in loss of pigment cells and dorsal root ganglion neurons. In contrast, late disruption of brain-specific Trpm7 after embryonic day 10.5 does not alter normal brain development. We developed induced pluripotent stem cells and neural stem (NS) cells in which Trpm7 disruption could be induced. Trpm7(-/-) NS cells retained the capacities of self-renewal and differentiation into neurons and astrocytes. During in vitro differentiation of induced pluripotent stem cells to NS cells, Trpm7 disruption prevents the formation of the NS cell monolayer. The in vivo and in vitro results demonstrate a temporal requirement for the Trpm7 channel kinase during embryogenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Runnels LW, Yue L, Clapham DE. TRP-PLIK, a bifunctional protein with kinase and ion channel activities. Science. 2001;291:1043–1047. - PubMed
-
- Runnels LW, Yue L, Clapham DE. The TRPM7 channel is inactivated by PIP(2) hydrolysis. Nat Cell Biol. 2002;4:329–336. - PubMed
-
- Yamaguchi H, Matsushita M, Nairn AC, Kuriyan J. Crystal structure of the atypical protein kinase domain of a TRP channel with phosphotransferase activity. Mol Cell. 2001;7:1047–1057. - PubMed
-
- Nadler MJ, et al. LTRPC7 is a Mg.ATP-regulated divalent cation channel required for cell viability. Nature. 2001;411:590–595. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

