The signaling pathway of Campylobacter jejuni-induced Cdc42 activation: Role of fibronectin, integrin beta1, tyrosine kinases and guanine exchange factor Vav2
- PMID: 22204307
- PMCID: PMC3286397
- DOI: 10.1186/1478-811X-9-32
The signaling pathway of Campylobacter jejuni-induced Cdc42 activation: Role of fibronectin, integrin beta1, tyrosine kinases and guanine exchange factor Vav2
Abstract
Background: Host cell invasion by the foodborne pathogen Campylobacter jejuni is considered as one of the primary reasons of gut tissue damage, however, mechanisms and key factors involved in this process are widely unclear. It was reported that small Rho GTPases, including Cdc42, are activated and play a role during invasion, but the involved signaling cascades remained unknown. Here we utilised knockout cell lines derived from fibronectin-/-, integrin-beta1-/-, focal adhesion kinase (FAK)-/- and Src/Yes/Fyn-/- deficient mice, and wild-type control cells, to investigate C. jejuni-induced mechanisms leading to Cdc42 activation and bacterial uptake.
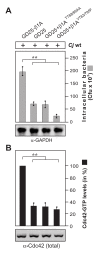
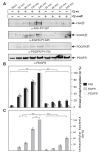
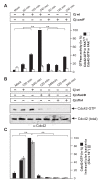
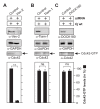
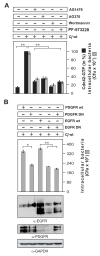
Results: Using high-resolution scanning electron microscopy, GTPase pulldowns, G-Lisa and gentamicin protection assays we found that each studied host factor is necessary for induction of Cdc42-GTP and efficient invasion. Interestingly, filopodia formation and associated membrane dynamics linked to invasion were only seen during infection of wild-type but not in knockout cells. Infection of cells stably expressing integrin-beta1 variants with well-known defects in fibronectin fibril formation or FAK signaling also exhibited severe deficiencies in Cdc42 activation and bacterial invasion. We further demonstrated that infection of wild-type cells induces increasing amounts of phosphorylated FAK and growth factor receptors (EGFR and PDGFR) during the course of infection, correlating with accumulating Cdc42-GTP levels and C. jejuni invasion over time. In studies using pharmacological inhibitors, silencing RNA (siRNA) and dominant-negative expression constructs, EGFR, PDGFR and PI3-kinase appeared to represent other crucial components upstream of Cdc42 and invasion. siRNA and the use of Vav1/2-/- knockout cells further showed that the guanine exchange factor Vav2 is required for Cdc42 activation and maximal bacterial invasion. Overexpression of certain mutant constructs indicated that Vav2 is a linker molecule between Cdc42 and activated EGFR/PDGFR/PI3-kinase. Using C. jejuni mutant strains we further demonstrated that the fibronectin-binding protein CadF and intact flagella are involved in Cdc42-GTP induction, indicating that the bacteria may directly target the fibronectin/integrin complex for inducing signaling leading to its host cell entry.
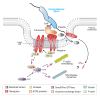
Conclusion: Collectively, our findings led us propose that C. jejuni infection triggers a novel fibronectin→integrin-beta1→FAK/Src→EGFR/PDGFR→PI3-kinase→Vav2 signaling cascade, which plays a crucial role for Cdc42 GTPase activity associated with filopodia formation and enhances bacterial invasion.
Figures




Similar articles
-
Major host factors involved in epithelial cell invasion of Campylobacter jejuni: role of fibronectin, integrin beta1, FAK, Tiam-1, and DOCK180 in activating Rho GTPase Rac1.Front Cell Infect Microbiol. 2011 Dec 12;1:17. doi: 10.3389/fcimb.2011.00017. eCollection 2011. Front Cell Infect Microbiol. 2011. PMID: 22919583 Free PMC article.
-
Role of the small Rho GTPases Rac1 and Cdc42 in host cell invasion of Campylobacter jejuni.Cell Microbiol. 2007 Oct;9(10):2431-44. doi: 10.1111/j.1462-5822.2007.00971.x. Epub 2007 May 23. Cell Microbiol. 2007. PMID: 17521326
-
Vav2 activates Rac1, Cdc42, and RhoA downstream from growth factor receptors but not beta1 integrins.Mol Cell Biol. 2000 Oct;20(19):7160-9. doi: 10.1128/MCB.20.19.7160-7169.2000. Mol Cell Biol. 2000. PMID: 10982832 Free PMC article.
-
Taking Control: Campylobacter jejuni Binding to Fibronectin Sets the Stage for Cellular Adherence and Invasion.Front Microbiol. 2020 Apr 9;11:564. doi: 10.3389/fmicb.2020.00564. eCollection 2020. Front Microbiol. 2020. PMID: 32328046 Free PMC article. Review.
-
Regulation of phosphorylation pathways by p21 GTPases. The p21 Ras-related Rho subfamily and its role in phosphorylation signalling pathways.Eur J Biochem. 1996 Dec 1;242(2):171-85. doi: 10.1111/j.1432-1033.1996.0171r.x. Eur J Biochem. 1996. PMID: 8973630 Review.
Cited by
-
Molecular Dissection of the Campylobacter jejuni CadF and FlpA Virulence Proteins in Binding to Host Cell Fibronectin.Microorganisms. 2020 Mar 11;8(3):389. doi: 10.3390/microorganisms8030389. Microorganisms. 2020. PMID: 32168837 Free PMC article.
-
Molecular Targets in Campylobacter Infections.Biomolecules. 2023 Feb 22;13(3):409. doi: 10.3390/biom13030409. Biomolecules. 2023. PMID: 36979344 Free PMC article. Review.
-
The fibronectin-binding motif within FlpA facilitates Campylobacter jejuni adherence to host cell and activation of host cell signaling.Emerg Microbes Infect. 2013 Oct;2(10):e65. doi: 10.1038/emi.2013.65. Epub 2013 Oct 9. Emerg Microbes Infect. 2013. PMID: 26038437 Free PMC article.
-
Evolution and Role of Proteases in Campylobacter jejuni Lifestyle and Pathogenesis.Biomolecules. 2023 Feb 8;13(2):323. doi: 10.3390/biom13020323. Biomolecules. 2023. PMID: 36830692 Free PMC article. Review.
-
Bacterial Outer Membrane Protein OmpX Regulates β1 Integrin and Epidermal Growth Factor Receptor (EGFR) Involved in Invasion of M-HeLa Cells by Serratia proteamaculans.Int J Mol Sci. 2021 Dec 9;22(24):13246. doi: 10.3390/ijms222413246. Int J Mol Sci. 2021. PMID: 34948042 Free PMC article.
References
-
- World Health Organization. Global burden of disease (GBD) 2002 estimates. WHO. Geneva, Switzerland; 2004. http://www.who.int/topics/global_burden_of_disease/en/
-
- Nachamkin I, Szymanski CM, Blaser MJ. Campylobacter. Washington, DC: ASM Press; 2008.
-
- Oyarzabal OA, Backert S. Microbial Food Safety: An Introduction. Heidelberg (Germany): Springer Verlag; 2012. in press .
-
- Blaser MJ, Engberg J. In: Campylobacter. Nachamkin I, Szymanski CM, Blaser MJ, editor. Washington, DC: ASM Press; 2008. pp. 99–121.
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

