Orf virus DNA vaccines expressing ORFV 011 and ORFV 059 chimeric protein enhances immunogenicity
- PMID: 22204310
- PMCID: PMC3269396
- DOI: 10.1186/1743-422X-8-562
Orf virus DNA vaccines expressing ORFV 011 and ORFV 059 chimeric protein enhances immunogenicity
Abstract
Background: ORFV attenuated live vaccines have been the main prophylactic measure against contagious ecthyma in sheep and goats in the last decades, which play an important role in preventing the outbreak of the disease. However, the available vaccines do not induce lasting immunity in sheep and goats. On the other hand, variation in the terminal genome of Orf virus vaccine strains during cell culture adaptation may affect the efficacy of a vaccine. Currently, there are no more effective antiviral treatments available for contagious ecthyma.
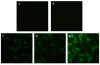
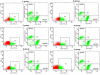
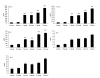
Results: We constructed three eukaryotic expression vectors pcDNA3.1-ORFV011, pcDNA3.1-ORFV059 and pcDNA3.1-ORFV011/ORFV059 and tested their immunogenicity in mouse model. High level expression of the recombinant proteins ORFV011, ORFV059 and ORFV011/ORFV059 was confirmed by western blotting analysis and indirect fluorescence antibody (IFA) tests. The ORFV-specific antibody titers and serum IgG1/IgG2a titers, the proliferation of lymphocytes and ORFV-specific cytokines (IL-2, IL-4, IL-6, IFN-γ, and TNF-α) were examined to evaluate the immune responses of the vaccinated mice. We found that mice inoculated with pcDNA3.1-ORFV 011/ORFV059 had significantly stronger immunological responses than those inoculated with pcDNA3.1-ORFV011, pcDNA3.1-ORFV059, or pcDNA3.1-ORFV011 plus pcDNA3.1-ORFV059. Compared to other vaccine plasmids immunized groups, pcDNA3.1-ORFV011/ORFV059 immunized group enhances immunogenicity.
Conclusions: We concluded that DNA vaccine pcDNA3.1-ORFV011/ORFV059 expressing ORFV011 and ORFV059 chemeric-proteins can significantly improve the potency of DNA vaccination and could be served as more effective and safe approach for new vaccines against ORFV.
Figures
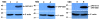
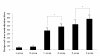
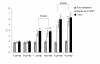
Similar articles
-
Contagious ecthyma in small ruminants: from etiology to vaccine challenges - a review.Vet Res Commun. 2025 Feb 24;49(2):115. doi: 10.1007/s11259-025-10677-0. Vet Res Commun. 2025. PMID: 39992468 Review.
-
Construction and Biological Characteristics of a Quadruple Gene-Deleted Strain of Orf Virus as a Vaccine Candidate.Viruses. 2025 May 27;17(6):760. doi: 10.3390/v17060760. Viruses. 2025. PMID: 40573351 Free PMC article.
-
Identification and characterization of monoclonal antibodies against the ORFV059 protein encoded by Orf virus.Virus Genes. 2012 Jun;44(3):429-40. doi: 10.1007/s11262-011-0710-9. Epub 2012 Jan 12. Virus Genes. 2012. PMID: 22237464
-
Evaluation of a recombinant major envelope protein (F1L) based indirect- ELISA for sero-diagnosis of orf in sheep and goats.J Virol Methods. 2018 Nov;261:112-120. doi: 10.1016/j.jviromet.2018.08.015. Epub 2018 Aug 24. J Virol Methods. 2018. PMID: 30149033
-
[Molecular characteristics and immune evasion strategies of ORFV: a review].Bing Du Xue Bao. 2012 May;28(3):278-84. Bing Du Xue Bao. 2012. PMID: 22764532 Review. Chinese.
Cited by
-
Polymorphism detection of promoter region of IFN-γ and IL-2 genes and their association with productive traits in Mazandaran native breeder fowls.J Genet. 2018 Sep;97(4):843-851. J Genet. 2018. PMID: 30262696
-
Contagious ecthyma in small ruminants: from etiology to vaccine challenges - a review.Vet Res Commun. 2025 Feb 24;49(2):115. doi: 10.1007/s11259-025-10677-0. Vet Res Commun. 2025. PMID: 39992468 Review.
-
Evaluation of recombinant extracellular enveloped virion protein candidates for the detection of serological responses to lumpy skin disease virus in cattle.Vet Q. 2025 Dec;45(1):1-13. doi: 10.1080/01652176.2025.2475989. Epub 2025 Mar 19. Vet Q. 2025. PMID: 40103407 Free PMC article.
-
Construction and Biological Characteristics of a Quadruple Gene-Deleted Strain of Orf Virus as a Vaccine Candidate.Viruses. 2025 May 27;17(6):760. doi: 10.3390/v17060760. Viruses. 2025. PMID: 40573351 Free PMC article.
-
Molecular identification and investigations of contagious ecthyma (Orf virus) in small ruminants, North west Ethiopia.BMC Vet Res. 2018 Jan 15;14(1):13. doi: 10.1186/s12917-018-1339-x. BMC Vet Res. 2018. PMID: 29334948 Free PMC article.
References
-
- Haig DM, Mercer AA. Ovine diseases. Orf. Vet Res. 1998;29:311–326. - PubMed
-
- De la Concha-Bermejillo A. In: Health Hazards in Veterinary Practice. 3. Farris R, Mahlow J, Newman E, Nix B, editor. American Veterinary Medical Association, Schaumburg; 1995. Poxviral diseases; pp. 55–56.
-
- Mazur C, Machado RD. The isolation and identification of the contagious ecthyma virus of caprines in cell cultures. Rev Microbiol São Paulo. 1990;21:127–130. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

