Alu elements: know the SINEs
- PMID: 22204421
- PMCID: PMC3334610
- DOI: 10.1186/gb-2011-12-12-236
Alu elements: know the SINEs
Abstract
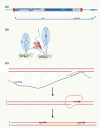
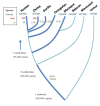
Alu elements are primate-specific repeats and comprise 11% of the human genome. They have wide-ranging influences on gene expression. Their contribution to genome evolution, gene regulation and disease is reviewed.
Figures



Similar articles
-
True Homoplasy of Retrotransposon Insertions in Primates.Syst Biol. 2019 May 1;68(3):482-493. doi: 10.1093/sysbio/syy076. Syst Biol. 2019. PMID: 30445649
-
Alu retrotransposition-mediated deletion.J Mol Biol. 2005 May 13;348(4):791-800. doi: 10.1016/j.jmb.2005.02.043. J Mol Biol. 2005. PMID: 15843013
-
Non-traditional Alu evolution and primate genomic diversity.J Mol Biol. 2002 Mar 8;316(5):1033-40. doi: 10.1006/jmbi.2001.5380. J Mol Biol. 2002. PMID: 11884141
-
Alu repeats and human genomic diversity.Nat Rev Genet. 2002 May;3(5):370-9. doi: 10.1038/nrg798. Nat Rev Genet. 2002. PMID: 11988762 Review.
-
[Alu elements in the human genome].Tidsskr Nor Laegeforen. 2004 Sep 23;124(18):2345-9. Tidsskr Nor Laegeforen. 2004. PMID: 15467796 Review. Norwegian.
Cited by
-
A study of transposable element-associated structural variations (TASVs) using a de novo-assembled Korean genome.Exp Mol Med. 2021 Apr;53(4):615-630. doi: 10.1038/s12276-021-00586-y. Epub 2021 Apr 8. Exp Mol Med. 2021. PMID: 33833373 Free PMC article.
-
High-Throughput Analysis of Global DNA Methylation Using Methyl-Sensitive Digestion.PLoS One. 2016 Oct 17;11(10):e0163184. doi: 10.1371/journal.pone.0163184. eCollection 2016. PLoS One. 2016. PMID: 27749902 Free PMC article.
-
RNAs as Regulators of Cellular Matchmaking.Front Mol Biosci. 2021 Apr 9;8:634146. doi: 10.3389/fmolb.2021.634146. eCollection 2021. Front Mol Biosci. 2021. PMID: 33898516 Free PMC article. Review.
-
Recent insights into crosstalk between genetic parasites and their host genome.Brief Funct Genomics. 2024 Jan 18;23(1):15-23. doi: 10.1093/bfgp/elac032. Brief Funct Genomics. 2024. PMID: 36307128 Free PMC article. Review.
-
A reference genome for the critically endangered woylie, Bettongia penicillata ogilbyi.GigaByte. 2021 Dec 10;2021:gigabyte35. doi: 10.46471/gigabyte.35. eCollection 2021. GigaByte. 2021. PMID: 36824341 Free PMC article.
References
-
- Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J, Devon K, Dewar K, Doyle M, FitzHugh W, Funke R, Gage D, Harris K, Heaford A, Howland J, Kann L, Lehoczky J, LeVine R, McEwan P, McKernan K, Meldrim J, Mesirov JP, Miranda C, Morris W, Naylor J, Raymond C, Rosetti M, Santos R, Sheridan A, Sougnez C. et al.Initial sequencing and analysis of the human genome. International Human Genome Sequencing Consortium. Nature. 2001;409:860–921. doi: 10.1038/35057062. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

