Cyclotides, a novel ultrastable polypeptide scaffold for drug discovery
- PMID: 22204428
- PMCID: PMC3330703
- DOI: 10.2174/138161211798999438
Cyclotides, a novel ultrastable polypeptide scaffold for drug discovery
Abstract
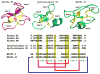
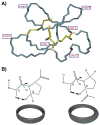
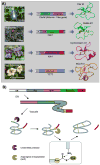
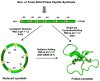
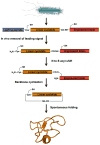
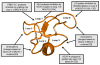
Cyclotides are a unique and growing family of backbone cyclized peptides that also contain a cystine knot motif built from six conserved cysteine residues. This unique circular backbone topology and knotted arrangement of three disulfide bonds makes them exceptionally stable to thermal, chemical, and enzymatic degradation compared to other peptides of similar size. Aside from the conserved residues forming the cystine knot, cyclotides have been shown to have high variability in their sequences. Consisting of over 160 known members, cyclotides have many biological activities, ranging from anti-HIV, antimicrobial, hemolytic, and uterotonic capabilities; additionally, some cyclotides have been shown to have cell penetrating properties. Originally discovered and isolated from plants, cyclotides can also be produced synthetically and recombinantly. The high sequence variability, stability, and cell penetrating properties of cyclotides make them potential scaffolds to be used to graft known active peptides or engineer peptide-based drug design. The present review reports recent findings in the biological diversity and therapeutic potential of natural and engineered cyclotides.
Figures
References
-
- Daly NL, Rosengren KJ, Craik DJ. Discovery, structure and biological activities of cyclotides. Adv Drug Deliv Rev. 2009;61:918–930. - PubMed
-
- Colgrave ML, Craik DJ. Thermal, chemical, and enzymatic stability of the cyclotide kalata B1: the importance of the cyclic cystine knot. Biochemistry. 2004;43:5965–5975. - PubMed
-
- Saether O, Craik DJ, Campbell ID, Sletten K, Juul J, Norman DG. Elucidation of the primary and three-dimensional structure of the uterotonic polypeptide kalata B1. Biochemistry. 1995;34:4147–4158. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

