Synthesis and biological activity of some 3-(4-(substituted)-piperazin-1-yl)cinnolines
- PMID: 22205089
- PMCID: PMC6268863
- DOI: 10.3390/molecules17010227
Synthesis and biological activity of some 3-(4-(substituted)-piperazin-1-yl)cinnolines
Abstract
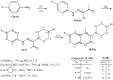
A new series of 6-substituted-4-methyl-3-(4-arylpiperazin-1-yl)cinnolines 8-10 were synthesized as potential antifungal agents via intramolecular cyclization of the respective 1-(2-arylhydrazono)-1-(4-arylpiperazin-1-yl)propan-2-ones 5-7, mediated by polyphosphoric acid (PPA). The amidrazones themselves were synthesized via direct interaction of the appropriate hydrazonoyl chlorides 4a-d with the corresponding N-substituted piperazine in the presence of triethylamine. The structures of the new prepared compounds were confirmed by elemental analyses, (1)H-NMR, (13)C-NMR, and ESI-HRMS spectral data. The antitumor, antibacterial, and antifungal activity of the newly synthesized compounds was evaluated.
Figures
Similar articles
-
Naturally occurring cyclohexane epoxides: sources, biological activities, and synthesis.Chem Rev. 2004 Jun;104(6):2857-99. doi: 10.1021/cr980013j. Chem Rev. 2004. PMID: 15186183 Review. No abstract available.
-
Synthesis and biological evaluation of (+/-)-dinemasone C and analogues.J Org Chem. 2010 Sep 3;75(17):6057-60. doi: 10.1021/jo101408s. J Org Chem. 2010. PMID: 20684519 Free PMC article.
-
Therapeutic Potential of Cinnoline Core: A Comprehensive Review.Mini Rev Med Chem. 2020;20(3):196-218. doi: 10.2174/1389557519666191011095858. Mini Rev Med Chem. 2020. PMID: 31660825 Review.
-
Synthesis, and antitumor activity of some N1-(coumarin-7-yl) amidrazones and related congeners.Molecules. 2011 May 24;16(5):4305-17. doi: 10.3390/molecules16054305. Molecules. 2011. PMID: 21610659 Free PMC article.
-
Enaminonitrile in heterocyclic synthesis: synthesis and antimicrobial evaluation of some new pyrazole, isoxazole and pyrimidine derivatives incorporating a benzothiazole moiety.Eur J Med Chem. 2009 Dec;44(12):4813-8. doi: 10.1016/j.ejmech.2009.07.024. Epub 2009 Jul 30. Eur J Med Chem. 2009. PMID: 19683840
Cited by
-
Tuneable access to indole, indolone, and cinnoline derivatives from a common 1,4-diketone Michael acceptor.Beilstein J Org Chem. 2020 Jul 17;16:1722-1731. doi: 10.3762/bjoc.16.144. eCollection 2020. Beilstein J Org Chem. 2020. PMID: 32733616 Free PMC article.
-
Cinnoline Scaffold-A Molecular Heart of Medicinal Chemistry?Molecules. 2019 Jun 18;24(12):2271. doi: 10.3390/molecules24122271. Molecules. 2019. PMID: 31216762 Free PMC article. Review.
-
Synthesis and Properties of 6-Aryl-4-azidocinnolines and 6-Aryl-4-(1,2,3-1H-triazol-1-yl)cinnolines.Molecules. 2019 Jun 27;24(13):2386. doi: 10.3390/molecules24132386. Molecules. 2019. PMID: 31252657 Free PMC article.
-
Understanding the aqueous chemistry of quinoline and the diazanaphthalenes: insight from DFT study.Heliyon. 2021 Jul 10;7(7):e07531. doi: 10.1016/j.heliyon.2021.e07531. eCollection 2021 Jul. Heliyon. 2021. PMID: 34296019 Free PMC article.
References
-
- Hennequin L.F., Thomas A.P., Johnstone C., Stokes E.S., Ple P.A., Lohman J.-J., Ogilve D.J., Dukes M., Wedge S.R., Curwen J.O., et al. Design and structure-activity relationship of a new class of potent VEGF receptor tyrosine kinase inhibitors. J. Med. Chem. 1999;42:5369–5389. - PubMed
-
- Bantick J., Hirst S., Perry M., Phillips E. Preparation of Fused Pyridazines as Allergy Inhibitors and Antiinflammatories. PCT Int. Appl. WO 9809969, 1998. Chem. Abstr. 1998;128:217375.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources