Synthesis and biological activity of some 3-(4-(substituted)-piperazin-1-yl)cinnolines
- PMID: 22205089
- PMCID: PMC6268863
- DOI: 10.3390/molecules17010227
Synthesis and biological activity of some 3-(4-(substituted)-piperazin-1-yl)cinnolines
Abstract
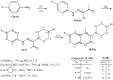
A new series of 6-substituted-4-methyl-3-(4-arylpiperazin-1-yl)cinnolines 8-10 were synthesized as potential antifungal agents via intramolecular cyclization of the respective 1-(2-arylhydrazono)-1-(4-arylpiperazin-1-yl)propan-2-ones 5-7, mediated by polyphosphoric acid (PPA). The amidrazones themselves were synthesized via direct interaction of the appropriate hydrazonoyl chlorides 4a-d with the corresponding N-substituted piperazine in the presence of triethylamine. The structures of the new prepared compounds were confirmed by elemental analyses, (1)H-NMR, (13)C-NMR, and ESI-HRMS spectral data. The antitumor, antibacterial, and antifungal activity of the newly synthesized compounds was evaluated.
Figures
References
-
- Hennequin L.F., Thomas A.P., Johnstone C., Stokes E.S., Ple P.A., Lohman J.-J., Ogilve D.J., Dukes M., Wedge S.R., Curwen J.O., et al. Design and structure-activity relationship of a new class of potent VEGF receptor tyrosine kinase inhibitors. J. Med. Chem. 1999;42:5369–5389. - PubMed
-
- Bantick J., Hirst S., Perry M., Phillips E. Preparation of Fused Pyridazines as Allergy Inhibitors and Antiinflammatories. PCT Int. Appl. WO 9809969, 1998. Chem. Abstr. 1998;128:217375.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources