Effect of titrated parenteral nutrition on body composition after allogeneic hematopoietic stem cell transplantation in children: a double-blind, randomized, multicenter trial
- PMID: 22205317
- PMCID: PMC3260068
- DOI: 10.3945/ajcn.111.026005
Effect of titrated parenteral nutrition on body composition after allogeneic hematopoietic stem cell transplantation in children: a double-blind, randomized, multicenter trial
Abstract
Background: Children undergoing hematopoietic stem cell transplantation (HSCT) often require parenteral nutrition (PN) to optimize caloric intake. Standard approaches to nutritional supplementation provide 130-150% of estimated energy expenditure, but resting energy expenditure (REE) may be lower than expected after HSCT. Provision of PN exceeding energy needs may lead to overfeeding and associated complications.
Objective: We conducted a blinded, randomized, controlled, multicenter trial in children undergoing HSCT to determine the effect on body composition of 2 different approaches of nutrition support: standard amounts of energy from PN (130-150% of REE) compared with PN titrated to match measured REE.
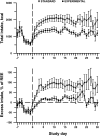
Design: Twenty-six children undergoing HSCT were randomly assigned to standard or titrated PN. Energy intake was monitored until day 30 after HSCT. Body-composition and anthropometric measures were obtained through day 100. The primary outcome variable was percentage body fat (%BF) measured by dual energy X-ray absorptiometry.
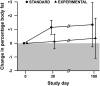
Results: The estimated change in %BF from baseline to day 30 was 1.2 ± 0.5% in the standard group and 0.1 ± 0.5% in the experimental group, but the overall time course of %BF did not differ significantly by treatment (P = 0.39 for time × treatment interaction). A profound loss of lean body mass (LBM) occurred in both groups during the intervention period and persisted through day 100.
Conclusions: Parenteral energy intake titrated to energy expenditure does not result in a lower accumulation of BF than does standard energy intake. Neither titrated nor standard PN regimens during HSCT preserve LBM. Alternative approaches to preserve LBM are needed. This trial is registered at clinicaltrials.gov as 00115258.
Trial registration: ClinicalTrials.gov NCT00115258.
Figures
Similar articles
-
Attenuation of resting energy expenditure following hematopoietic SCT in children.Bone Marrow Transplant. 2012 Oct;47(10):1301-6. doi: 10.1038/bmt.2012.19. Epub 2012 Feb 20. Bone Marrow Transplant. 2012. PMID: 22343669 Free PMC article. Clinical Trial.
-
A multi-center, randomized, controlled trial of parenteral nutrition titrated to resting energy expenditure in children undergoing hematopoietic stem cell transplantation ("PNTREE"): rationale and design.Contemp Clin Trials. 2010 Mar;31(2):157-64. doi: 10.1016/j.cct.2009.12.002. Epub 2010 Jan 3. Contemp Clin Trials. 2010. PMID: 20004739 Free PMC article. Clinical Trial.
-
Higher compared with lower dietary protein during an energy deficit combined with intense exercise promotes greater lean mass gain and fat mass loss: a randomized trial.Am J Clin Nutr. 2016 Mar;103(3):738-46. doi: 10.3945/ajcn.115.119339. Epub 2016 Jan 27. Am J Clin Nutr. 2016. PMID: 26817506 Clinical Trial.
-
Nutritional and Post-Transplantation Outcomes of Enteral versus Parenteral Nutrition in Pediatric Hematopoietic Stem Cell Transplantation: A Systematic Review of Randomized and Nonrandomized Studies.Biol Blood Marrow Transplant. 2019 Aug;25(8):e252-e259. doi: 10.1016/j.bbmt.2019.02.023. Epub 2019 Mar 1. Biol Blood Marrow Transplant. 2019. PMID: 30826462
-
Enteral versus Parenteral Nutrition as Nutritional Support after Allogeneic Hematopoietic Stem Cell Transplantation: a Systematic Review and Meta-Analysis.Transplant Cell Ther. 2021 Feb;27(2):180.e1-180.e8. doi: 10.1016/j.jtct.2020.11.006. Epub 2020 Dec 13. Transplant Cell Ther. 2021. PMID: 33830034
Cited by
-
Body Composition in Children with Chronic Illness: Accuracy of Bedside Assessment Techniques.J Pediatr. 2017 Nov;190:56-62. doi: 10.1016/j.jpeds.2017.07.045. J Pediatr. 2017. PMID: 29144272 Free PMC article.
-
Bone loss and vitamin D deficiency in children undergoing hematopoietic cell transplantation.Pediatr Blood Cancer. 2015 Apr;62(4):687-92. doi: 10.1002/pbc.25370. Epub 2015 Jan 28. Pediatr Blood Cancer. 2015. PMID: 25630874 Free PMC article. Clinical Trial.
-
Attenuation of resting energy expenditure following hematopoietic SCT in children.Bone Marrow Transplant. 2012 Oct;47(10):1301-6. doi: 10.1038/bmt.2012.19. Epub 2012 Feb 20. Bone Marrow Transplant. 2012. PMID: 22343669 Free PMC article. Clinical Trial.
-
Serum citrulline as a biomarker of gastrointestinal function during hematopoietic cell transplantation in children.J Pediatr Gastroenterol Nutr. 2014 Jun;58(6):709-14. doi: 10.1097/MPG.0000000000000335. J Pediatr Gastroenterol Nutr. 2014. PMID: 24614125 Free PMC article.
References
-
- Muscaritoli M, Grieco G, Capria S, Iori AP, Rossi Fanelli F. Nutrition and metabolic support in patients undergoing bone marrow transplantation. Am J Clin Nutr 2002;75:183–90 - PubMed
-
- Weisdorf S, Hofland C, Sharp H, Teasley K, Schissel K, McGlave P, Ramsay N, Kersey J. Total parenteral nutrition in bone marrow transplantation: a clinical evaluation. J Pediatr Gastroenterol Nutr 1984;3:95–100 - PubMed
-
- Weisdorf SA, Lysne J, Wind D, Haake R, Sharp H, Goldman A, Schissel K, McGlave PB, Ramsay NK, Kersey JH. Positive effect of prophylactic total parenteral nutrition on long-term outcome of bone marrow transplantation. Transplantation 1987;43:833–8 - PubMed
-
- Kerner JA, Jr, Hurwitz M. Parenteral nutrition. : Duggan C, Watkins J, Walker WA, Nutrition in pediatrics: basic science and clinical applications. 4th ed. Hamilton, Ontario: BC Decker, 2008:777–93
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

