Body fat abnormality in HIV-infected children and adolescents living in Europe: prevalence and risk factors
- PMID: 22205436
- PMCID: PMC3433033
- DOI: 10.1097/QAI.0b013e31824330cb
Body fat abnormality in HIV-infected children and adolescents living in Europe: prevalence and risk factors
Abstract
Objectives: To estimate the prevalence of and identify risk factors for lipodystrophy syndrome (LS) and body fat abnormality in a population of HIV-infected children and adolescents.
Design: Cross-sectional observational study.
Methods: HIV-infected subjects aged 2-18 years were recruited from 15 HIV centers in Belgium, Italy, and Poland between January 2007 and December 2008. Standardized assessments by the patient's long-term clinician were performed to establish the presence of abnormality. Risk factors were explored in logistic regression models for fat abnormality outcomes and LS (abnormality plus dyslipidemia).
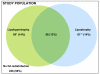
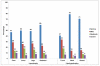
Results: Among 426 subjects (70% white), median age was 12.2 years (interquartile range: 9.0-15.0 years) and median duration of antiretroviral therapy was 5.2 years (interquartile range: 2.2-8.8 years). Prevalence was 57% (n = 235) for LS and 42% (n = 176) for fat abnormality; 90 subjects with abnormality were affected in ≥3 locations. Lipoatrophy occurred in 28% (n = 117) of subjects and lipohypertrophy in 27% (n = 115), most commonly in the face and trunk, respectively. In multivariable analysis, white ethnicity, body mass index, ritonavir/lopinavir, and nonnucleoside reverse transcriptase inhibitors were each associated with an increased risk of LS (P < 0.05). White ethnicity, history of Centers for Disease Control and Prevention-defined disease, and stavudine were associated with risk of lipoatrophy (P < 0.05). Increased risk of lipohypertrophy was associated with body mass index and prior HIV disease.
Conclusions: Fat abnormality was prevalent in close to half of children and adolescents, who had accumulated long treatment durations. Risk of fat abnormality was associated with specific drugs, including stavudine and ritonavir, and other variables. Our results underline the importance of continued surveillance of children treated with antiretroviral therapy.
Figures
References
-
- Heath CV, Hogg RS, Chan KJ, Harris M, Montessori V, O’Shaughnessy MV, et al. Lipodystrophy-associated morphological cholesterol and triglyceride abnormalities in a population-based HIVAIDS treatment database. AIDS. 2001;15:231–239. - PubMed
-
- Carr A, Samaras K, Thorsidottir A, Kaufman GR, Chisholm DJ, DA C. Diagnosis, prediction, and natural course of HIV-1 protease-inhibitor-associated lipodystrophy, hyperlipidaemia, and diabetes mellitus: A cohort study. The Lancet. 1999;353:2093–2099. - PubMed
-
- Saves M, F R, Capeau J, Rozenbaum W, Ragnaud J-M, Peronne C, et al. Factors related to lipodystrophy and metabolic alterations in patients with human immunodeficiency virus infection receiving highly active antiretroviral therapy. Clinical Infectious Diseases. 2002;34:1396–1405. - PubMed
-
- Chene G, Angelini E, Cotte L, Lang J-M, Morlat P, Rancinan C, et al. Role of long-term nucleoside-analogue therapy in lipodystrophy and metabolic disorders in human immunodeficiency virus-infected patients. Clinical Infectious Diseases. 2002;34:649–657. - PubMed
-
- Shlay JC, Visnegarwala F, Bartsch G, Wang J, Peng G, El-Sadr WM, et al. Body composition and metabolic changes in antiretroviral-naive patients randomized to didanosine and stavudine vs. abacavir and lamivudine. Journal of Acquired Immune Deficiency Syndromes. 2005;38:147–155. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

