Immunological response to parenteral vaccination with recombinant hepatitis B virus surface antigen virus-like particles expressing Helicobacter pylori KatA epitopes in a murine H. pylori challenge model
- PMID: 22205658
- PMCID: PMC3272931
- DOI: 10.1128/CVI.05295-11
Immunological response to parenteral vaccination with recombinant hepatitis B virus surface antigen virus-like particles expressing Helicobacter pylori KatA epitopes in a murine H. pylori challenge model
Abstract
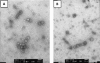
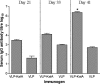
Virus-like particles (VLPs) based on the small envelope protein of hepatitis B virus (HBsAg-S) are immunogenic at the B- and T-cell level. In this study, we inserted overlapping sequences encoding the carboxy terminus of the Helicobacter pylori katA gene product into HBsAg-S. The HBsAg-S-KatA fusion proteins were able to assemble into secretion-competent VLPs (VLP-KatA). The VLP-KatA proteins were able to induce KatA-specific antibodies in immunized mice. The mean total IgG antibody titers 41 days post-primary immunization with VLP-KatA (2.3 × 10(3)) were significantly greater (P < 0.05) than those observed for vaccination with VLP alone (5.2 × 10(2)). Measurement of IgG isotypes revealed responses to both IgG1 and IgG2a (mean titers, 9.0 × 10(4) and 2.6 × 10(4), respectively), with the IgG2a response to vaccination with VLP-KatA being significantly higher than that for mice immunized with KatA alone (P < 0.05). Following challenge of mice with H. pylori, a significantly reduced bacterial load in the gastric mucosa was observed (P < 0.05). This is the first report describing the use of VLPs as a delivery vehicle for H. pylori antigens.
Figures


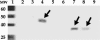


Similar articles
-
Immunological response of recombinant H. pylori multi-epitope vaccine with different vaccination strategies.Int J Clin Exp Pathol. 2014 Sep 15;7(10):6559-66. eCollection 2014. Int J Clin Exp Pathol. 2014. PMID: 25400734 Free PMC article.
-
Recombinant herpesvirus glycoprotein G improves the protective immune response to Helicobacter pylori vaccination in a mouse model of disease.PLoS One. 2014 May 2;9(5):e96563. doi: 10.1371/journal.pone.0096563. eCollection 2014. PLoS One. 2014. PMID: 24794215 Free PMC article.
-
Oral Immunization with a Multivalent Epitope-Based Vaccine, Based on NAP, Urease, HSP60, and HpaA, Provides Therapeutic Effect on H. pylori Infection in Mongolian gerbils.Front Cell Infect Microbiol. 2017 Aug 4;7:349. doi: 10.3389/fcimb.2017.00349. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28824883 Free PMC article.
-
Helicobacter pylori vaccine strategies--triggering a gut reaction.Immunol Today. 2000 Dec;21(12):615-9. doi: 10.1016/s0167-5699(00)01753-9. Immunol Today. 2000. PMID: 11114421 Review.
-
DNA-mediated immunization to the hepatitis B surface antigen. Activation and entrainment of the immune response.Ann N Y Acad Sci. 1995 Nov 27;772:64-76. doi: 10.1111/j.1749-6632.1995.tb44732.x. Ann N Y Acad Sci. 1995. PMID: 8546414 Review.
Cited by
-
Immunogenicity of Leishmania-derived hepatitis B small surface antigen particles exposing highly conserved E2 epitope of hepatitis C virus.Microb Cell Fact. 2016 Apr 13;15:62. doi: 10.1186/s12934-016-0460-4. Microb Cell Fact. 2016. PMID: 27075377 Free PMC article.
-
Hepatitis B Virus (HBV) Subviral Particles as Protective Vaccines and Vaccine Platforms.Viruses. 2020 Jan 21;12(2):126. doi: 10.3390/v12020126. Viruses. 2020. PMID: 31973017 Free PMC article. Review.
-
Immunogenicity of Wild Type and Mutant Hepatitis B Surface Antigen Virus-like Particles (VLPs) in Mice with Pre-Existing Immunity against the Wild Type Vector.Viruses. 2023 Jan 23;15(2):313. doi: 10.3390/v15020313. Viruses. 2023. PMID: 36851527 Free PMC article.
-
An intranasal influenza virus vector vaccine protects against Helicobacter pylori in mice.J Virol. 2024 Mar 19;98(3):e0192323. doi: 10.1128/jvi.01923-23. Epub 2024 Feb 15. J Virol. 2024. PMID: 38358289 Free PMC article.
-
Chimeric hepatitis B surface antigen virus-like particles expressing the strep A epitope p*17 elicit a humoral immune response in mice.Heliyon. 2024 May 4;10(9):e30606. doi: 10.1016/j.heliyon.2024.e30606. eCollection 2024 May 15. Heliyon. 2024. PMID: 38765111 Free PMC article.
References
-
- Abimiku AG, Dolby JM. 1988. Cross-protection of infant mice against intestinal colonisation by Campylobacter jejuni: importance of heat-labile serotyping (Lior) antigens. J. Med. Microbiol. 26:265–268 - PubMed
-
- Ada GF. 1990. The immunological basis of vaccine development. Semin. Virol. 1:3–11
-
- Akhiani AA, Lycke N. 2002. T cell involvement in experimental Helicobacter pylori specific protective immunity. Recent Res. Dev. Immunol. 4:259
-
- Arora S, Czinn SJ. 2005. Vaccination as a method of preventing H. pylori-associated gastric cancer. Cancer Epidemiol. Biomarkers Prev. 14:1890–1891 - PubMed
-
- Chan K, et al. 2002. Eradication of and risk of peptic ulcers in patients starting long-term treatment with non-steroidal anti-inflammatory drugs: a randomised trial. Lancet 359:9–13 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

