Influenza A virus infection results in a robust, antigen-responsive, and widely disseminated Foxp3+ regulatory T cell response
- PMID: 22205730
- PMCID: PMC3302292
- DOI: 10.1128/JVI.05685-11
Influenza A virus infection results in a robust, antigen-responsive, and widely disseminated Foxp3+ regulatory T cell response
Abstract
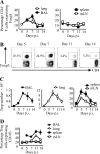
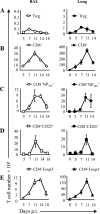
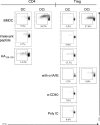
Foxp3(+) CD4(+) regulatory T cells (Tregs) represent a highly suppressive T cell subset with well-characterized immunosuppressive effects during immune homeostasis and chronic infections, although the role of these cells in acute viral infections is poorly understood. The present study sought to examine the induction of Foxp3(+) CD4(+) Tregs in a nonlethal murine model of pulmonary viral infection by the use of the prototypical respiratory virus influenza A. We establish that influenza A virus infection results in a robust Foxp3(+) CD4(+) T cell response and that regulatory T cell induction at the site of inflammation precedes the effector T cell response. Induced Foxp3(+) CD4(+) T cells are highly suppressive ex vivo, demonstrating that influenza virus-induced Foxp3(+) CD4(+) T cells are phenotypically regulatory. Influenza A virus-induced regulatory T cells proliferate vigorously in response to influenza virus antigen, are disseminated throughout the site of infection and primary and secondary lymphoid organs, and retain Foxp3 expression in vitro, suggesting that acute viral infection is capable of inducing a foreign-antigen-specific Treg response. The ability of influenza virus-induced regulatory T cells to suppress antigen-specific CD4(+) and CD8(+) T cell proliferation and cytokine production correlates closely to their ability to respond to influenza virus antigens, suggesting that virus-induced Tregs are capable of attenuating effector responses in an antigen-dependent manner. Collectively, these data demonstrate that primary acute viral infection is capable of inducing a robust, antigen-responsive, and suppressive regulatory T cell response.
Figures



References
-
- Belkaid Y, Piccirillo CA, Mendez S, Shevach EM, Sacks DL. 2002. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature 420:502–507 - PubMed
-
- Belz GT, Stevenson PG, Doherty PC. 2000. Contemporary analysis of MHC-related immunodominance hierarchies in the CD8+ T cell response to influenza A viruses. J. Immunol. 165:2404–2409 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

