CD4 T cells promote CD8 T cell immunity at the priming and effector site during viral encephalitis
- PMID: 22205741
- PMCID: PMC3302259
- DOI: 10.1128/JVI.06797-11
CD4 T cells promote CD8 T cell immunity at the priming and effector site during viral encephalitis
Abstract
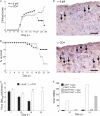
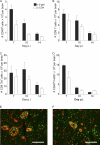
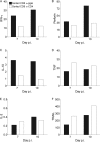
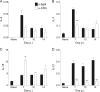
CD4 T cell activation during peripheral infections not only is essential in inducing protective CD8 T cell memory but also promotes CD8 T cell function and survival. However, the contributions of CD4 T cell help to antiviral CD8 T cell immunity during central nervous system (CNS) infection are not well established. Encephalitis induced by the sublethal coronavirus JHMV was used to identify when CD4 T cells regulate CD8 T cell responses following CNS infection. Peripheral expansion of virus-specific CD8 T cells was impaired when CD4 T cells were ablated prior to infection but not at 4 days postinfection. Delayed CD4 T cell depletion abrogated CD4 T cell recruitment to the CNS but only slightly diminished CD8 T cell recruitment. Nevertheless, the absence of CNS CD4 T cells was associated with reduced gamma interferon (IFN-γ) and granzyme B expression by infiltrating CD8 T cells, increased CD8 T cell apoptosis, and impaired control of infectious virus. CD4 T cell depletion subsequent to CD4 T cell CNS migration restored CD8 T cell activity and virus control. Analysis of γc-dependent cytokine expression indicated interleukin-21 (IL-21) as a primary candidate optimizing CD8 T cell activity within the CNS. These results demonstrate that CD4 T cells play critical roles in both enhancing peripheral activation of CD8 T cells and prolonging their antiviral function within the CNS. The data highlight the necessity for temporally and spatially distinct CD4 T cell helper functions in sustaining CD8 T cell activity during CNS infection.
Figures





References
-
- Altfeld M, Rosenberg ES. 2000. The role of CD4+ T helper cells in the cytotoxic T lymphocyte response to HIV-1. Current Opin. Immunol. 12:375–380 - PubMed
-
- Bergmann CC, Altman JD, Hinton D, Stohlman SA. 1999. Inverted immunodominance and impaired cytolytic function of CD8+ T cells during viral persistence in the central nervous system. J. Immunol. 163:3379–3387 - PubMed
-
- Castellino F, et al. 2006. Chemokines enhance immunity by guiding naive CD8+ T cells to sites of CD4+ T cell-dendritic cell interaction. Nature 440:890–895 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

