An inducible cytochrome P450 3A4-dependent vitamin D catabolic pathway
- PMID: 22205755
- PMCID: PMC3310418
- DOI: 10.1124/mol.111.076356
An inducible cytochrome P450 3A4-dependent vitamin D catabolic pathway
Abstract
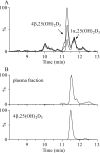
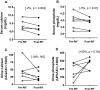
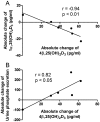
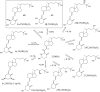
Vitamin D(3) is critical for the regulation of calcium and phosphate homeostasis. In some individuals, mineral homeostasis can be disrupted by long-term therapy with certain antiepileptic drugs and the antimicrobial agent rifampin, resulting in drug-induced osteomalacia, which is attributed to vitamin D deficiency. We now report a novel CYP3A4-dependent pathway, the 4-hydroxylation of 25-hydroxyvitamin D(3) (25OHD(3)), the induction of which may contribute to drug-induced vitamin D deficiency. The metabolism of 25OHD(3) was fully characterized in vitro. CYP3A4 was the predominant source of 25OHD(3) hydroxylation by human liver microsomes, with the formation of 4β,25-dihydroxyvitamin D(3) [4β,25(OH)(2)D(3)] dominating (V(max)/K(m) = 0.85 ml · min(-1) · nmol enzyme(-1)). 4β,25(OH)(2)D(3) was found in human plasma at concentrations comparable to that of 1α,25-dihydroxyvitamin D(3), and its formation rate in a panel of human liver microsomes was strongly correlated with CYP3A4 content and midazolam hydroxylation activity. Formation of 4β,25(OH)(2)D(3) in primary human hepatocytes was induced by rifampin and inhibited by CYP3A4-specific inhibitors. Short-term treatment of healthy volunteers (n = 6) with rifampin selectively induced CYP3A4-dependent 4β,25(OH)(2)D(3), but not CYP24A1-dependent 24R,25-dihydroxyvitamin D(3) formation, and altered systemic mineral homeostasis. Our results suggest that CYP3A4-dependent 25OHD(3) metabolism may play an important role in the regulation of vitamin D(3) in vivo and in the etiology of drug-induced osteomalacia.
Figures



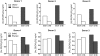


References
-
- Alexander BH, Dimler RJ, Mehltretter CL. (1951) d-Galactosan(1,4)α(1,6): its structure and resistance to periodate oxidation. J Am Chem Soc 73:4658–4659
-
- Araya Z, Hosseinpour F, Bodin K, Wikvall K. (2003) Metabolism of 25-hydroxyvitamin D3 by microsomal and mitochondrial vitamin D3 25-hydroxylases (CYP2D25 and CYP27A1): a novel reaction by CYP27A1. Biochim Biophys Acta 1632:40–47 - PubMed
-
- Brodie MJ, Boobis AR, Dollery CT, Hillyard CJ, Brown DJ, MacIntyre I, Park BK. (1980) Rifampicin and vitamin D metabolism. Clin Pharmacol Ther 27:810–814 - PubMed
-
- Brodie MJ, Boobis AR, Hillyard CJ, Abeyasekera G, Stevenson JC, MacIntyre I, Park BK. (1982) Effect of rifampicin and isoniazid on vitamin D metabolism. Clin Pharmacol Ther 32:525–530 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

