Matrix-assisted laser desorption ionization-time of flight mass spectrometry-based functional assay for rapid detection of resistance against β-lactam antibiotics
- PMID: 22205812
- PMCID: PMC3295133
- DOI: 10.1128/JCM.05737-11
Matrix-assisted laser desorption ionization-time of flight mass spectrometry-based functional assay for rapid detection of resistance against β-lactam antibiotics
Abstract
Resistance against β-lactam antibiotics is a growing challenge for managing severe bacterial infections. The rapid and cost-efficient determination of β-lactam resistance is an important prerequisite for the choice of an adequate antibiotic therapy. β-Lactam resistance is based mainly on the expression/overexpression of β-lactamases, which destroy the central β-lactam ring of these drugs by hydrolysis. Hydrolysis corresponds to a mass shift of +18 Da, which can be easily detected by matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS). Therefore, a MALDI-TOF MS-based assay was set up to investigate different enterobacteria for resistance against different β-lactam antibiotics: ampicillin, piperacillin, cefotaxime, ceftazidime, ertapenem, imipenem, and meropenem. β-Lactamases are enzymes that have a high turnover rate. Therefore, hydrolysis can be detected by MALDI-TOF MS already after a few hours of incubation of the bacteria to be tested with the given antibiotic. The comparison of the MS-derived data with the data from the routine procedure revealed identical classification of the bacteria according to sensitivity and resistance. The MALDI-TOF MS-based assay delivers the results on the same day. The approved routine procedures require at least an additional overnight incubation.
Figures


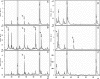

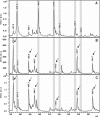
References
-
- Baechle D, et al. 2007. Towards stable diagnostic setups in clinical proteomics: absolute quantitation of peptide biomarkers using MALDI-TOF-MS. Proteomics 1:1280–1284
-
- Bou G. 2007. Minimum inhibitory concentration (MIC) analysis and susceptibility testing of MRSA. Methods Mol. Biol. 391:29–49 - PubMed
-
- Bush K. 2010. Alarming β-lactamase-mediated resistance in multidrug-resistant Enterobacteriaceae. Curr. Opin. Microbiol. 13:558–564 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

