Evaluation of three electronic noses for detecting incipient wood decay
- PMID: 22205858
- PMCID: PMC3244004
- DOI: 10.3390/s100201062
Evaluation of three electronic noses for detecting incipient wood decay
Abstract
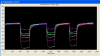
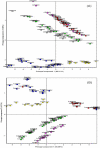
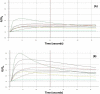
Tree assessment methodologies, currently used to evaluate the structural stability of individual urban trees, usually involve a visual analysis followed by measurements of the internal soundness of wood using various instruments that are often invasive, expensive, or inadequate for use within the urban environment. Moreover, most conventional instruments do not provide an adequate evaluation of decay that occurs in the root system. The intent of this research was to evaluate the possibility of integrating conventional tools, currently used for assessments of decay in urban trees, with the electronic nose-a new innovative tool used in diverse fields and industries for various applications such as quality control in manufacturing, environmental monitoring, medical diagnoses, and perfumery. Electronic-nose (e-nose) technologies were tested for the capability of detecting differences in volatile organic compounds (VOCs) released by wood decay fungi and wood from healthy and decayed trees. Three e-noses, based on different types of operational technologies and analytical methods, were evaluated independently (not directly compared) to determine the feasibility of detecting incipient decays in artificially-inoculated wood. All three e-nose devices were capable of discriminating between healthy and artificially-inoculated, decayed wood with high levels of precision and confidence. The LibraNose quartz microbalance (QMB) e-nose generally provided higher levels of discrimination of sample unknowns, but not necessarily more accurate or effective detection than the AromaScan A32S conducting polymer and PEN3 metal-oxide (MOS) gas sensor e-noses for identifying and distinguishing woody samples containing different agents of wood decay. However, the conducting polymer e-nose had the greater advantage for identifying unknowns from diverse woody sample types due to the associated software capability of utilizing prior-developed, application-specific reference libraries with aroma pattern-recognition and neural-net training algorithms.
Keywords: electronic aroma detection; tree hazard assessment; urban landscape tree species; wood-rotting fungi.
Figures








References
-
- Smiley E.T., Fraedrich B.R., Fengler P. Hazard tree inspection, evaluation, and management. In: Kuser J., editor. Urban and Community Forestry in the Northeast. Kluwer Academic/Plenum; New York, NY, USA: 2007. pp. 277–294.
-
- Rayner A.D.M., Boddy L. Fungal decomposition of Wood: Its Biology and Ecology. Wiley; Chichester, UK: 1988.
-
- Mattheck C., Breloer H. Field guide for visual tree assessment. Arboric. J. 1994;18:1–23.
-
- Wilson A.D., Lester D.G., Oberle C.S. Development of conductive polymer analysis for the rapid detection and identification of phytopathogenic microbes. Phytopathology. 2004;94:419–431. - PubMed
-
- Korpi A., Kasanen J.P., Alarie Y., Kosma V.M., Pasanen A.L. Sensory irritating potency of some microbial volatile organic compounds (MVOCs) and a mixture of five MVOCs. Arch. Environ. Health. 1999;54:347–352. - PubMed

