Recognition and degradation of plant cell wall polysaccharides by two human gut symbionts
- PMID: 22205877
- PMCID: PMC3243724
- DOI: 10.1371/journal.pbio.1001221
Recognition and degradation of plant cell wall polysaccharides by two human gut symbionts
Abstract
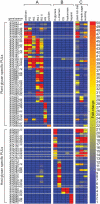
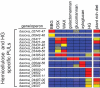
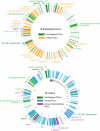
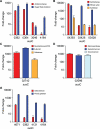
Symbiotic bacteria inhabiting the human gut have evolved under intense pressure to utilize complex carbohydrates, primarily plant cell wall glycans in our diets. These polysaccharides are not digested by human enzymes, but are processed to absorbable short chain fatty acids by gut bacteria. The Bacteroidetes, one of two dominant bacterial phyla in the adult gut, possess broad glycan-degrading abilities. These species use a series of membrane protein complexes, termed Sus-like systems, for catabolism of many complex carbohydrates. However, the role of these systems in degrading the chemically diverse repertoire of plant cell wall glycans remains unknown. Here we show that two closely related human gut Bacteroides, B. thetaiotaomicron and B. ovatus, are capable of utilizing nearly all of the major plant and host glycans, including rhamnogalacturonan II, a highly complex polymer thought to be recalcitrant to microbial degradation. Transcriptional profiling and gene inactivation experiments revealed the identity and specificity of the polysaccharide utilization loci (PULs) that encode individual Sus-like systems that target various plant polysaccharides. Comparative genomic analysis indicated that B. ovatus possesses several unique PULs that enable degradation of hemicellulosic polysaccharides, a phenotype absent from B. thetaiotaomicron. In contrast, the B. thetaiotaomicron genome has been shaped by increased numbers of PULs involved in metabolism of host mucin O-glycans, a phenotype that is undetectable in B. ovatus. Binding studies of the purified sensor domains of PUL-associated hybrid two-component systems in conjunction with transcriptional analyses demonstrate that complex oligosaccharides provide the regulatory cues that induce PUL activation and that each PUL is highly specific for a defined cell wall polymer. These results provide a view of how these species have diverged into different carbohydrate niches by evolving genes that target unique suites of available polysaccharides, a theme that likely applies to disparate bacteria from the gut and other habitats.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Comment in
-
How bacteria turn fiber into food.PLoS Biol. 2011 Dec;9(12):e1001227. doi: 10.1371/journal.pbio.1001227. Epub 2011 Dec 20. PLoS Biol. 2011. PMID: 22205880 Free PMC article. No abstract available.
References
-
- Flint H. J, Bayer E. A, Rincon M. T, Lamed R, White B. A. Polysaccharide utilization by gut bacteria: potential for new insights from genomic analysis. Nat Rev Microbiol. 2008;6:121–131. - PubMed
-
- McNeil N. I. The contribution of the large intestine to energy supplies in man. Am J Clin Nutr. 1984;39:338–342. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- BB/F014163/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- R01 DK030292/DK/NIDDK NIH HHS/United States
- DK084214/DK/NIDDK NIH HHS/United States
- F32 AI073060/AI/NIAID NIH HHS/United States
- T32 HD07409/HD/NICHD NIH HHS/United States
- T32 HG000045/HG/NHGRI NIH HHS/United States
- K01 DK084214/DK/NIDDK NIH HHS/United States
- R37 DK030292/DK/NIDDK NIH HHS/United States
- K22 HG000045/HG/NHGRI NIH HHS/United States
- HG00045/HG/NHGRI NIH HHS/United States
- T32 GM007200/GM/NIGMS NIH HHS/United States
- DK30292/DK/NIDDK NIH HHS/United States
- GM07200/GM/NIGMS NIH HHS/United States
- T32 HD007409/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

