The endocrine disruptor mono-(2-ethylhexyl) phthalate affects the differentiation of human liposarcoma cells (SW 872)
- PMID: 22205965
- PMCID: PMC3244402
- DOI: 10.1371/journal.pone.0028750
The endocrine disruptor mono-(2-ethylhexyl) phthalate affects the differentiation of human liposarcoma cells (SW 872)
Abstract
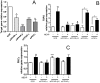
Esters of phthalic acid (phthalates) are largely used in industrial plastics, medical devices, and pharmaceutical formulations. They are easily released from plastics into the environment and can be found in measurable levels in human fluids. Phthalates are agonists for peroxisome proliferator-activated receptors (PPARs), through which they regulate translocator protein (TSPO; 18 kDa) transcription in a tissue-specific manner. TSPO is a drug- and cholesterol-binding protein involved in mitochondrial respiration, steroid formation, and cell proliferation. TSPO has been shown to increase during differentiation and decrease during maturation in mouse adipocytes. The purpose of this study was to establish the effect of mono-(2-ethylhexyl) phthalate (MEHP) on the differentiation of human SW 872 preadipocyte cells, and examine the role of TSPO in the process. After 4 days of treatment with 10 µM MEHP, we observed changes in the transcription of acetyl-CoA carboxylase alpha, adenosine triphosphate citrate lyase, glucose transporters 1 and 4, and the S100 calcium binding protein B, all of which are markers of preadipocyte differentiation. These observed gene expression changes coincided with a decrease in cellular proliferation without affecting cellular triglyceride content. Taken together, these data suggest that MEHP exerts a differentiating effect on human preadipocytes. Interestingly, MEHP was able to temporarily increase TSPO mRNA levels through the PPAR-α and β/δ pathways. These results suggest that TSPO can be considered an important player in the differentiation process itself, or alternatively a factor whose presence is essential for adipocyte development.
Conflict of interest statement
Figures
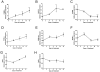



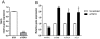

Similar articles
-
Activation of PPARalpha and PPARgamma by environmental phthalate monoesters.Toxicol Sci. 2003 Aug;74(2):297-308. doi: 10.1093/toxsci/kfg145. Epub 2003 Jun 12. Toxicol Sci. 2003. PMID: 12805656
-
The endocrine disruptor mono-(2-ethylhexyl) phthalate promotes adipocyte differentiation and induces obesity in mice.Biosci Rep. 2012 Dec;32(6):619-29. doi: 10.1042/BSR20120042. Biosci Rep. 2012. PMID: 22953781 Free PMC article.
-
Mono(2-ethylhexyl)phthalate and mono-n-butyl phthalate activation of peroxisome proliferator activated-receptors alpha and gamma in breast.Toxicol Lett. 2006 Jun 1;163(3):224-34. doi: 10.1016/j.toxlet.2005.11.001. Epub 2005 Dec 1. Toxicol Lett. 2006. PMID: 16326050
-
Fetal origin of endocrine dysfunction in the adult: the phthalate model.J Steroid Biochem Mol Biol. 2013 Sep;137:5-17. doi: 10.1016/j.jsbmb.2013.01.007. Epub 2013 Jan 17. J Steroid Biochem Mol Biol. 2013. PMID: 23333934 Review.
-
Mechanisms of phthalate ester toxicity in the female reproductive system.Environ Health Perspect. 2003 Feb;111(2):139-45. doi: 10.1289/ehp.5658. Environ Health Perspect. 2003. PMID: 12573895 Free PMC article. Review.
Cited by
-
In utero growth restriction and catch-up adipogenesis after developmental di (2-ethylhexyl) phthalate exposure cause glucose intolerance in adult male rats following a high-fat dietary challenge.J Nutr Biochem. 2015 Nov;26(11):1208-20. doi: 10.1016/j.jnutbio.2015.05.012. Epub 2015 Jun 20. J Nutr Biochem. 2015. PMID: 26188368 Free PMC article.
-
Regulation of Translocator Protein 18 kDa (TSPO) Expression in Rat and Human Male Germ Cells.Int J Mol Sci. 2016 Sep 6;17(9):1486. doi: 10.3390/ijms17091486. Int J Mol Sci. 2016. PMID: 27608010 Free PMC article.
-
Mediating Roles of PPARs in the Effects of Environmental Chemicals on Sex Steroids.PPAR Res. 2017;2017:3203161. doi: 10.1155/2017/3203161. Epub 2017 Jul 27. PPAR Res. 2017. PMID: 28819354 Free PMC article. Review.
-
Hydromethanolic Extracts from Adansonia digitata L. Edible Parts Positively Modulate Pathophysiological Mechanisms Related to the Metabolic Syndrome.Molecules. 2020 Jun 21;25(12):2858. doi: 10.3390/molecules25122858. Molecules. 2020. PMID: 32575811 Free PMC article.
-
Translocator protein 18 kDa (TSPO) is regulated in white and brown adipose tissue by obesity.PLoS One. 2013 Nov 18;8(11):e79980. doi: 10.1371/journal.pone.0079980. eCollection 2013. PLoS One. 2013. PMID: 24260329 Free PMC article.
References
-
- Halden RU. Plastics and health risks. Annu Rev Public Health. 2010;31:179–94. - PubMed
-
- Fromme H, Lahrz T, Piloty M, Gebhart H, Oddoy A, et al. Occurrence of phthalates and musk fragrances in indoor air and dust from apartments and kindergartens in Berlin (Germany). Indoor Air. 2004;14:188–95. - PubMed
-
- Agency for Toxic Substances and Disease Registry (ATSDR) 1993. Toxicological Profile for Di(2-ethylhexyl)phthalate. Public Health Service, U.S. Department of Health and Human Services, Atlanta, GA. Available: http://www.atsdr.cdc.gov/substances/toxsubstance.asp?toxid=65. Accessed 2011 Jul 14.
-
- Health Canada report. DEHP in Medical Devices. 2001. Available: http://www.hc-sc.gc.ca/dhp-mps/md-im/activit/sci-consult/dehp/sapdehp_re.... Accessed 2011 Jul 14.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

