Selective cytotoxicity against human osteosarcoma cells by a novel synthetic C-1 analogue of 7-deoxypancratistatin is potentiated by curcumin
- PMID: 22205968
- PMCID: PMC3244407
- DOI: 10.1371/journal.pone.0028780
Selective cytotoxicity against human osteosarcoma cells by a novel synthetic C-1 analogue of 7-deoxypancratistatin is potentiated by curcumin
Abstract
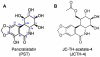
The natural compound pancratistatin (PST) is a non-genotoxic inducer of apoptosis in a variety of cancers. It exhibits cancer selectivity as non-cancerous cells are markedly less sensitive to PST. Nonetheless, PST is not readily synthesized and is present in very low quantities in its natural source to be applied clinically. We have previously synthesized and evaluated several synthetic analogues of 7-deoxypancratistatin, and found that JC-TH-acetate-4 (JCTH-4), a C-1 acetoxymethyl analogue, possessed similar apoptosis inducing activity compared to PST. In this study, notoriously chemoresistant osteosarcoma (OS) cells (Saos-2, U-2 OS) were substantially susceptible to JCTH-4-induced apoptosis through mitochondrial targeting; JCTH-4 induced collapse of mitochondrial membrane potential (MMP), increased reactive oxygen species (ROS) production in isolated mitochondria, and caused release of apoptosis inducing factor (AIF) and endonuclease G (EndoG) from isolated mitochondria. Furthermore, JCTH-4 selectively induced autophagy in OS cells. Additionally, we investigated the combinatory effect of JCTH-4 with the natural compound curcumin (CC), a compound found in turmeric spice, previously shown to possess antiproliferative properties. CC alone had no observable effect on Saos-2 and U-2 OS cells. However, when present with JCTH-4, CC was able to enhance the cytotoxicity of JCTH-4 selectively in OS cells. Such cytotoxicity by JCTH-4 alone and in combination with CC was not observed in normal human osteoblasts (HOb) and normal human fetal fibroblasts (NFF). Therefore, this report illustrates a new window in combination therapy, utilizing a novel synthetic analogue of PST with the natural compound CC, for the treatment of OS.
Conflict of interest statement
Figures







Similar articles
-
A novel synthetic C-1 analogue of 7-deoxypancratistatin induces apoptosis in p53 positive and negative human colorectal cancer cells by targeting the mitochondria: enhancement of activity by tamoxifen.Invest New Drugs. 2012 Jun;30(3):1012-27. doi: 10.1007/s10637-011-9668-7. Epub 2011 Apr 15. Invest New Drugs. 2012. PMID: 21494837
-
Enhancement of apoptotic and autophagic induction by a novel synthetic C-1 analogue of 7-deoxypancratistatin in human breast adenocarcinoma and neuroblastoma cells with tamoxifen.J Vis Exp. 2012 May 30;(63):3586. doi: 10.3791/3586. J Vis Exp. 2012. PMID: 22688195 Free PMC article.
-
Exploiting mitochondrial and oxidative vulnerabilities with a synthetic analog of pancratistatin in combination with piperlongumine for cancer therapy.FASEB J. 2018 Jan;32(1):417-430. doi: 10.1096/fj.201700275R. Epub 2017 Sep 19. FASEB J. 2018. PMID: 28928246
-
Pathological and Pharmacological Roles of Mitochondrial Reactive Oxygen Species in Malignant Neoplasms: Therapies Involving Chemical Compounds, Natural Products, and Photosensitizers.Molecules. 2020 Nov 11;25(22):5252. doi: 10.3390/molecules25225252. Molecules. 2020. PMID: 33187225 Free PMC article. Review.
-
Apoptosis-Inducing Effects of Amaryllidaceae Alkaloids.Curr Med Chem. 2016;23(2):161-85. doi: 10.2174/0929867323666151118121124. Curr Med Chem. 2016. PMID: 26577925 Review.
Cited by
-
Curcuma Contra Cancer? Curcumin and Hodgkin's Lymphoma.Cancer Growth Metastasis. 2013 Aug 8;6:35-52. doi: 10.4137/CGM.S11113. eCollection 2013. Cancer Growth Metastasis. 2013. PMID: 24665206 Free PMC article. Review.
-
Biochemistry, Safety, Pharmacological Activities, and Clinical Applications of Turmeric: A Mechanistic Review.Evid Based Complement Alternat Med. 2020 May 10;2020:7656919. doi: 10.1155/2020/7656919. eCollection 2020. Evid Based Complement Alternat Med. 2020. PMID: 32454872 Free PMC article. Review.
-
The Multifaceted Therapeutic Mechanisms of Curcumin in Osteosarcoma: State-of-the-Art.J Oncol. 2021 Oct 11;2021:3006853. doi: 10.1155/2021/3006853. eCollection 2021. J Oncol. 2021. PMID: 34671398 Free PMC article. Review.
-
Elaborating the role of natural products-induced autophagy in cancer treatment: achievements and artifacts in the state of the art.Biomed Res Int. 2015;2015:934207. doi: 10.1155/2015/934207. Epub 2015 Mar 3. Biomed Res Int. 2015. PMID: 25821829 Free PMC article. Review.
-
New small molecules targeting apoptosis and cell viability in osteosarcoma.PLoS One. 2015 Jun 3;10(6):e0129058. doi: 10.1371/journal.pone.0129058. eCollection 2015. PLoS One. 2015. PMID: 26039064 Free PMC article.
References
-
- Aggarwal B, Kumar A, Bharti A. Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res. 2003;23(1A):363–398. - PubMed
-
- Singh S. From exotic spice to modern drug? Cell. 2007;130:765–768. - PubMed
-
- Kuttan R, Bhanumathy P, Nirmala K, George M. Potential anticancer activity of turmeric (Curcuma longa). Cancer Lett. 1985;29(2):197–202. - PubMed
-
- Walters DK, Muff R, Langsam B, Born W, Fuchs B. Cytotoxic effects of curcumin on osteosarcoma cell lines. Invest New Drugs. 2008;26(4):289–97. Epub 2007 Dec 11. - PubMed
-
- Arndt CA, Crist WM. Common musculoskeletal tumors of childhood and adolescence. N Engl J Med. 1999;341:342–352. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

