CCL5 neutralization restricts cancer growth and potentiates the targeting of PDGFRβ in colorectal carcinoma
- PMID: 22205974
- PMCID: PMC3243667
- DOI: 10.1371/journal.pone.0028842
CCL5 neutralization restricts cancer growth and potentiates the targeting of PDGFRβ in colorectal carcinoma
Abstract
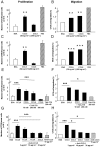
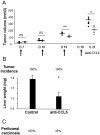
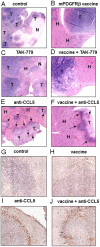
Increased CCL5 levels are markers of an unfavourable outcome in patients with melanoma, breast, cervical, prostate, gastric or pancreatic cancer. Here, we have assessed the role played by CCL5/CCR5 interactions in the development of colon cancer. To do so, we have examined a number of human colorectal carcinoma clinical specimens and found CCL5 and its receptors over-expressed within primary as well as liver and pulmonary metastases of patients compared to healthy tissues. In vitro, CCL5 increased the growth and migratory responses of colon cancer cells from both human and mouse origins. In addition, systemic treatment of mice with CCL5-directed antibodies reduced the extent of development of subcutaneous colon tumors, of liver metastases and of peritoneal carcinosis. Consistently, we found increased numbers of CD45-immunoreactive cells within the stroma of the remaining lesions as well as at the interface with the healthy tissue. In contrast, selective targeting of CCR5 through administration of TAK-779, a CCR5 antagonist, only partially compromised colon cancer progression. Furthermore, CCL5 neutralization rendered the tumors more sensitive to a PDGFRβ-directed strategy in mice, this combination regimen offering the greatest protection against liver metastases and suppressing macroscopic peritoneal carcinosis. Collectively, our data demonstrate the involvement of CCL5 in the pathogenesis of colorectal carcinoma and point to its potential value as a therapeutic target.
Conflict of interest statement
Figures



References
-
- Mueller MM, Fusenig NE. Friends or foes - bipolar effects of the tumour stroma in cancer. Nat Rev Cancer. 2004;4(11):839–49. - PubMed
-
- Tlsty TD, Coussens LM. Tumor stroma and regulation of cancer development. Annu Rev Pathol. 2006;1:119–50. - PubMed
-
- Ito M, Yoshida K, Kyo E, Ayhan A, Nakayama H, et al. Expression of several growth factors and their receptor genes in human colon carcinomas. Virchows Arch B Cell Pathol Incl Mol Pathol. 1990;59(3):173–8. - PubMed
-
- De Jong KP, Stellema R, Karrenbeld A, Koudstaal J, Gouw AS, et al. Clinical relevance of transforming growth factor alpha, epidermal growth factor receptor, p53, and Ki67 in colorectal liver metastases and corresponding primary tumors. Hepatology. 1998;28(4):971–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

