APC/C(Cdh1)-mediated degradation of the F-box protein NIPA is regulated by its association with Skp1
- PMID: 22205987
- PMCID: PMC3243670
- DOI: 10.1371/journal.pone.0028998
APC/C(Cdh1)-mediated degradation of the F-box protein NIPA is regulated by its association with Skp1
Abstract
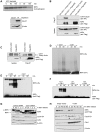
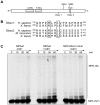
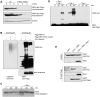
NIPA (Nuclear Interaction Partner of Alk kinase) is an F-box like protein that targets nuclear Cyclin B1 for degradation. Integrity and therefore activity of the SCF(NIPA) E3 ligase is regulated by cell-cycle-dependent phosphorylation of NIPA, restricting substrate ubiquitination to interphase. Here we show that phosphorylated NIPA is degraded in late mitosis in an APC/C(Cdh1)-dependent manner. Binding of the unphosphorylated form of NIPA to Skp1 interferes with binding to the APC/C-adaptor protein Cdh1 and therefore protects unphosphorylated NIPA from degradation in interphase. Our data thus define a novel mode of regulating APC/C-mediated ubiquitination.
Conflict of interest statement
Figures

Similar articles
-
NIPA defines an SCF-type mammalian E3 ligase that regulates mitotic entry.Cell. 2005 Jul 15;122(1):45-57. doi: 10.1016/j.cell.2005.04.034. Cell. 2005. PMID: 16009132
-
Multisite phosphorylation of nuclear interaction partner of ALK (NIPA) at G2/M involves cyclin B1/Cdk1.J Biol Chem. 2007 Jun 1;282(22):15965-72. doi: 10.1074/jbc.M610819200. Epub 2007 Mar 27. J Biol Chem. 2007. PMID: 17389604
-
Electron microscopy structure of human APC/C(CDH1)-EMI1 reveals multimodal mechanism of E3 ligase shutdown.Nat Struct Mol Biol. 2013 Jul;20(7):827-35. doi: 10.1038/nsmb.2593. Epub 2013 May 26. Nat Struct Mol Biol. 2013. PMID: 23708605 Free PMC article.
-
Methods to measure ubiquitin-dependent proteolysis mediated by the anaphase-promoting complex.Methods. 2006 Jan;38(1):39-51. doi: 10.1016/j.ymeth.2005.07.005. Methods. 2006. PMID: 16343932 Review.
-
Don't skip the G1 phase: how APC/CCdh1 keeps SCFSKP2 in check.Cell Cycle. 2004 Jul;3(7):850-2. Epub 2004 Jul 14. Cell Cycle. 2004. PMID: 15190201 Review.
Cited by
-
Loss of the Fanconi anemia-associated protein NIPA causes bone marrow failure.J Clin Invest. 2020 Jun 1;130(6):2827-2844. doi: 10.1172/JCI126215. J Clin Invest. 2020. PMID: 32338640 Free PMC article.
-
NIPA (Nuclear Interaction Partner of ALK) Is Crucial for Effective NPM-ALK Mediated Lymphomagenesis.Front Oncol. 2022 May 13;12:875117. doi: 10.3389/fonc.2022.875117. eCollection 2022. Front Oncol. 2022. PMID: 35646639 Free PMC article.
-
The Negative Cross-Talk between SAG/RBX2/ROC2 and APC/C E3 Ligases in Regulation of Cell Cycle Progression and Drug Resistance.Cell Rep. 2020 Sep 8;32(10):108102. doi: 10.1016/j.celrep.2020.108102. Cell Rep. 2020. PMID: 32905768 Free PMC article.
-
Association of genetic variants with atrial fibrillation.Biomed Rep. 2016 Feb;4(2):178-182. doi: 10.3892/br.2015.551. Epub 2015 Dec 2. Biomed Rep. 2016. PMID: 26893834 Free PMC article.
-
The Insulin Receptor Adaptor IRS2 is an APC/C Substrate That Promotes Cell Cycle Protein Expression and a Robust Spindle Assembly Checkpoint.Mol Cell Proteomics. 2020 Sep;19(9):1450-1467. doi: 10.1074/mcp.RA120.002069. Epub 2020 Jun 18. Mol Cell Proteomics. 2020. PMID: 32554797 Free PMC article.
References
-
- Sumara I, Maerki S, Peter M. E3 ubiquitin ligases and mitosis: embracing the complexity. Trends Cell Biol. 2008;18:84–94. - PubMed
-
- Golan A, Yudkovsky Y, Hershko A. The cyclin-ubiquitin ligase activity of cyclosome/APC is jointly activated by protein kinases Cdk1-cyclin B and Plk. J Biol Chem. 2002;277:15552–15557. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

