Tortuosity triggers platelet activation and thrombus formation in microvessels
- PMID: 22206421
- PMCID: PMC3489919
- DOI: 10.1115/1.4005478
Tortuosity triggers platelet activation and thrombus formation in microvessels
Abstract
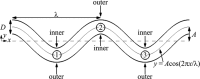
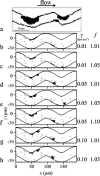
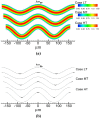
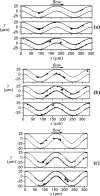
Tortuous blood vessels are often seen in humans in association with thrombosis, atherosclerosis, hypertension, and aging. Vessel tortuosity can cause high fluid shear stress, likely promoting thrombosis. However, the underlying physical mechanisms and microscale processes are poorly understood. Accordingly, the objectives of this study were to develop and use a new computational approach to determine the effects of venule tortuosity and fluid velocity on thrombus initiation. The transport, collision, shear-induced activation, and receptor-ligand adhesion of individual platelets in thrombus formation were simulated using discrete element method. The shear-induced activation model assumed that a platelet became activated if it experienced a shear stress above a relative critical shear stress or if it contacted an activated platelet. Venules of various levels of tortuosity were simulated for a mean flow velocity of 0.10 cm s(-1), and a tortuous arteriole was simulated for a mean velocity of 0.47 cm s(-1). Our results showed that thrombus was initiated at inner walls in curved regions due to platelet activation in agreement with experimental studies. Increased venule tortuosity modified fluid flow to hasten thrombus initiation. Compared to the same sized venule, flow in the arteriole generated a higher amount of mural thrombi and platelet activation rate. The results suggest that the extent of tortuosity is an important factor in thrombus initiation in microvessels.
Figures

Similar articles
-
Effect of Red Blood Cells on Platelet Activation and Thrombus Formation in Tortuous Arterioles.Front Bioeng Biotechnol. 2013 Dec 3;1:18. doi: 10.3389/fbioe.2013.00018. eCollection 2013. Front Bioeng Biotechnol. 2013. PMID: 25022613 Free PMC article.
-
Platelet size and density affect shear-induced thrombus formation in tortuous arterioles.Phys Biol. 2013 Oct;10(5):056003. doi: 10.1088/1478-3975/10/5/056003. Epub 2013 Aug 23. Phys Biol. 2013. PMID: 23974300 Free PMC article.
-
Numerical Simulation of Thrombotic Occlusion in Tortuous Arterioles.J Cardiol Cardiovasc Med. 2017;2(1):95-111. Epub 2017 Dec 6. J Cardiol Cardiovasc Med. 2017. PMID: 29327739 Free PMC article.
-
Thrombus Formation at High Shear Rates.Annu Rev Biomed Eng. 2017 Jun 21;19:415-433. doi: 10.1146/annurev-bioeng-071516-044539. Epub 2017 Apr 24. Annu Rev Biomed Eng. 2017. PMID: 28441034 Review.
-
Modeling thrombus formation and growth.Biotechnol Bioeng. 2017 Oct;114(10):2154-2172. doi: 10.1002/bit.26343. Epub 2017 Jun 26. Biotechnol Bioeng. 2017. PMID: 28542700 Review.
Cited by
-
Improving the 3D Printability of Sugar Glass to Engineer Sacrificial Vascular Templates.3D Print Addit Manuf. 2023 Oct 1;10(5):869-886. doi: 10.1089/3dp.2021.0147. Epub 2023 Oct 10. 3D Print Addit Manuf. 2023. PMID: 37886415 Free PMC article.
-
Mechanical buckling of arterioles in collateral development.J Theor Biol. 2013 Jan 7;316:42-8. doi: 10.1016/j.jtbi.2012.09.029. Epub 2012 Sep 30. J Theor Biol. 2013. PMID: 23034307 Free PMC article.
-
Obstacles in haemocompatibility testing.Scientifica (Cairo). 2013;2013:392584. doi: 10.1155/2013/392584. Epub 2013 May 7. Scientifica (Cairo). 2013. PMID: 24278774 Free PMC article. Review.
-
Simulation of the microscopic process during initiation of stent thrombosis.Comput Biol Med. 2015 Jan;56:182-91. doi: 10.1016/j.compbiomed.2014.11.006. Epub 2014 Nov 15. Comput Biol Med. 2015. PMID: 25437232 Free PMC article.
-
Models of Shear-Induced Platelet Activation and Numerical Implementation With Computational Fluid Dynamics Approaches.J Biomech Eng. 2022 Apr 1;144(4):040801. doi: 10.1115/1.4052460. J Biomech Eng. 2022. PMID: 34529037 Free PMC article.
References
-
- Wolf, Y. G. , Tillich, M. , Lee, W. A. , Rubin, G. D. , Fogarty, T. J. , and Zarins, C. K. , 2001, “Impact of Aortoiliac Tortuosity on Endovascular Repair of Abdominal Aortic Aneurysms: Evaluation of 3D Computer-Based Assessment,” J. Vasc. Surg., 34(4), pp. 594–599.10.1067/mva.2001.118586 - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

