Multi-tyrosine kinase inhibitors in preclinical studies for pediatric CNS AT/RT: Evidence for synergy with Topoisomerase-I inhibition
- PMID: 22206574
- PMCID: PMC3278350
- DOI: 10.1186/1475-2867-11-44
Multi-tyrosine kinase inhibitors in preclinical studies for pediatric CNS AT/RT: Evidence for synergy with Topoisomerase-I inhibition
Abstract
Background: Currently, Atypical Teratoid Rhabdoid Tumor (AT/RT) constitutes one of the most difficult to treat malignancies in pediatrics. Hence, new knowledge of potential targets for therapeutics and the development of novel treatment approaches are urgently needed. We have evaluated the presence of cytokine pathways and the effects of two clinically available multi-tyrosine kinase inhibitors for cytotoxicity, target modulation and drug combinability against AT/RT cell lines.
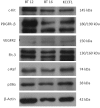
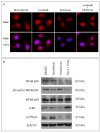
Results: AT/RT cell lines expressed measurable quantities of VEGF, FGF, PDGF and SDF-1, although the absolute amounts varied between the cell lines. The targeted receptor tyrosine kinase inhibitor sorafenib inhibited the key signaling molecule Erk, which was activated following the addition of own conditioned media, suggesting the existence of autocrine/paracrine growth stimulatory pathways. The multi-tyrosine kinase inhibitors sorafenib and sunitinib also showed significant growth inhibition of AT/RT cells and their activity was enhanced by combination with the topoisomerase inhibitor, irinotecan. The loss of cytoplasmic NF-kappa-B in response to irinotecan was diminished by sorafenib, providing evidence for a possible benefit for this drug combination.
Conclusions: In addition to previously described involvement of insulin like growth factor (IGF) family of cytokines, a multitude of other growth factors may contribute to the growth and survival of AT/RT cells. However, consistent with the heterogeneous nature of this tumor, quantitative and qualitative differences may exist among different tumor samples. Multi-tyrosine kinase inhibitors appear to have effective antitumor activity against all cell lines studied. In addition, the target modulation studies and drug combinability data provide the groundwork for additional studies and support the evaluation of these agents in future treatment protocols.
Figures



References
-
- Dufour C, Beaugrand A, Le Deley MC, Bourdeaut F, André N, Leblond P, Bertozzi AI, Frappaz D, Rialland X, Fouyssac F, Edan C, Grill J, Quidot M, Varlet P. Clinicopathologic prognostic factors in childhood atypical teratoid and rhabdoid tumor of the central nervous system: A multicenter study. Cancer. 2011. Electronically published ahead of print. - PubMed
-
- Bouvier C, De Paula AM, Fernandez C, Quilichini B, Scavarda D, Gentet JC, Figarella-Branger D. Atypical teratoid/rhabdoid tumor: 7-year event-free survival with gross total resection and radiotherapy in a 7-year-old boy. Childs Nerv Syst. 2008;24:143–147. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

