Oocyte specific oolemmal SAS1B involved in sperm binding through intra-acrosomal SLLP1 during fertilization
- PMID: 22206759
- PMCID: PMC3307385
- DOI: 10.1016/j.ydbio.2011.12.021
Oocyte specific oolemmal SAS1B involved in sperm binding through intra-acrosomal SLLP1 during fertilization
Abstract
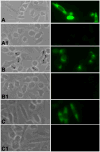
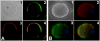
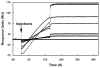
Molecular mechanisms by which fertilization competent acrosome-reacted sperm bind to the oolemma remain uncharacterized. To identify oolemmal binding partner(s) for sperm acrosomal ligands, affinity panning was performed with mouse oocyte lysates using sperm acrosomal protein, SLLP1 as a target. An oocyte specific membrane metalloproteinase, SAS1B (Sperm Acrosomal SLLP1 Binding), was identified as a SLLP1 binding partner. cDNA cloning revealed six SAS1B splice variants, each containing a zinc binding active site and a putative transmembrane domain, with signal peptides in three variants. SAS1B transcripts were ovary specific. SAS1B protein was first detected in early secondary follicles in day 3 ovaries. Immunofluorescence localized SAS1B to the microvillar oolemma of M2 oocytes. After fertilization, SAS1B decreased on the oolemma and became virtually undetectable in blastocysts. In transfected CHO-K1 cells SAS1B localized to the surface of unpermeabilized cells. Recombinant and native SLLP1 co-localized with SAS1B to the microvillar domain of ovulated M2 oocytes. Molecular interactions between mouse SLLP1 and SAS1B were demonstrated by surface plasmon resonance, far-western, yeast two-hybrid, recombinant- and native-co-IP analyses. SAS1B bound to SLLP1 with high affinity. SAS1B had protease activity, and SAS1B protein or antibody significantly inhibited fertilization. SAS1B knockout female mice showed a 34% reduction in fertility. The study identified SAS1B-SLLP1 as a pair of novel sperm-egg binding partners involving the oolemma and intra-acrosomal compartment during fertilization.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
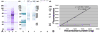






References
-
- Alfieri JA, Martin AD, Takeda J, Kondoh G, Myles DG, Primakoff P. Infertility in female mice with an oocyte-specific knockout of GPI-anchored proteins. J Cell Sci. 2003;116:2149–2155. - PubMed
-
- Almeida EA, Huovila AP, Sutherland AE, Stephens LE, Calarco PG, Shaw LM, Mercurio AM, Sonnenberg A, Primakoff P, Myles DG. Mouse egg integrin α6β1 functions as a sperm receptor. Cell. 1995;81:1095–1104. - PubMed
-
- Cho C, Bunch DO, Faure JE, Goulding EH, Eddy EM, Primakoff P, Myles DG. Fertilization defects in sperm from mice lacking fertilin β. Science. 1998;281:1857–1859. - PubMed
-
- Cohen DJ, Ellerman DA, Cuasnicu PS. Mammalian sperm-egg fusion: evidence that epididymal protein DE plays a role in mouse gamete fusion. Biol Reprod. 2000;63:462–468. - PubMed
-
- Coonrod SA, Naaby-Hansen S, Shetty J, Shibahara H, Chen M, White JM, Herr JC. Treatment of mouse oocytes with PI-PLC releases 70-kDa (pI 5) and 35- to 45-kDa (pI 5.5) protein clusters from the egg surface and inhibits sperm-oolemma binding and fusion. Dev Biol. 1999;207:334–349. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

