Ex vivo enrichment of circulating anti-tumor T cells from both cutaneous and ocular melanoma patients: clinical implications for adoptive cell transfer therapy
- PMID: 22207316
- PMCID: PMC3401505
- DOI: 10.1007/s00262-011-1179-z
Ex vivo enrichment of circulating anti-tumor T cells from both cutaneous and ocular melanoma patients: clinical implications for adoptive cell transfer therapy
Abstract
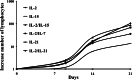
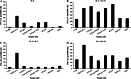
Tumor-infiltrating lymphocytes (TILs) have been successfully used for adoptive cell transfer (ACT) immunotherapy; however, due to their scarce availability, this therapy is possible for a limited fraction of cutaneous melanoma patients. We assessed whether an effective protocol for ex vivo T-cell expansion from peripheral blood mononuclear cells (PBMCs), suitable for ACT of both cutaneous and ocular melanoma patients, could be identified. PBMCs from both cutaneous and ocular melanoma patients were stimulated in vitro with autologous, irradiated melanoma cells (mixed lymphocyte tumor cell culture; MLTCs) in the presence of IL-2 and IL-15 followed by the rapid expansion protocol (REP). The functional activity of these T lymphocytes was characterized and compared with that of TILs. In addition, the immune infiltration in vivo of ocular melanoma lesions was analyzed. An efficient in vitro MLTC expansion of melanoma reactive T cells was achieved from all PBMC's samples obtained in 7 cutaneous and ocular metastatic melanoma patients. Large numbers of melanoma-specific T cells could be obtained when the REP protocol was applied to these MLTCs. Most MLTCs were enriched in non-terminally differentiated T(EM) cells homogeneously expressing co-stimulatory molecules (e.g., NKG2D, CD28, CD134, CD137). A similar pattern of anti-tumor activity, in association with a more variable expression of co-stimulatory molecules, was detected on short-term in vitro cultured TILs isolated from the same patients. In these ocular melanoma patients, we observed an immune infiltrate with suppressive characteristics and a low rate of ex vivo growing TILs (28.5% of our cases). Our MLTC protocol overcomes this limitation, allowing the isolation of T lymphocytes with effector functions even in these patients. Thus, anti-tumor circulating PBMC-derived T cells could be efficiently isolated from melanoma patients by our novel ex vivo enrichment protocol. This protocol appears suitable for ACT studies of cutaneous and ocular melanoma patients.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




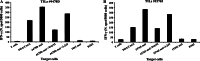

Similar articles
-
Co-stimulation through 4-1BB/CD137 improves the expansion and function of CD8(+) melanoma tumor-infiltrating lymphocytes for adoptive T-cell therapy.PLoS One. 2013;8(4):e60031. doi: 10.1371/journal.pone.0060031. Epub 2013 Apr 1. PLoS One. 2013. PMID: 23560068 Free PMC article.
-
IDO and galectin-3 hamper the ex vivo generation of clinical grade tumor-specific T cells for adoptive cell therapy in metastatic melanoma.Cancer Immunol Immunother. 2017 Jul;66(7):913-926. doi: 10.1007/s00262-017-1995-x. Epub 2017 Apr 11. Cancer Immunol Immunother. 2017. PMID: 28401257 Free PMC article.
-
Expansion and characterization of human melanoma tumor-infiltrating lymphocytes (TILs).PLoS One. 2010 Nov 10;5(11):e13940. doi: 10.1371/journal.pone.0013940. PLoS One. 2010. PMID: 21085676 Free PMC article.
-
Optimizing TIL therapy for uveal melanoma: lessons learned and unlearned from cutaneous melanoma.Immunotherapy. 2025 Mar;17(4):283-291. doi: 10.1080/1750743X.2025.2478808. Epub 2025 Mar 18. Immunotherapy. 2025. PMID: 40098478 Free PMC article. Review.
-
Achievements and challenges of adoptive T cell therapy with tumor-infiltrating or blood-derived lymphocytes for metastatic melanoma: what is needed to achieve standard of care?Cancer Immunol Immunother. 2014 Oct;63(10):1081-91. doi: 10.1007/s00262-014-1580-5. Epub 2014 Aug 7. Cancer Immunol Immunother. 2014. PMID: 25099366 Free PMC article. Review.
Cited by
-
Absence of CD4 T-cell help provides a robust CD8 T-cell response while inducing effective memory in a preclinical model of melanoma.Immunotherapy. 2012 May;4(5):477-81. doi: 10.2217/imt.12.39. Immunotherapy. 2012. PMID: 22642330 Free PMC article.
-
Generation of Pure Highly Functional Human Anti-Tumor Specific Cytotoxic T Lymphocytes With Stem Cell-Like Memory Features for Melanoma Immunotherapy.Front Immunol. 2021 Sep 8;12:674276. doi: 10.3389/fimmu.2021.674276. eCollection 2021. Front Immunol. 2021. PMID: 34566953 Free PMC article.
-
Lipid Nanoparticles for mRNA Delivery to Enhance Cancer Immunotherapy.Molecules. 2022 Aug 31;27(17):5607. doi: 10.3390/molecules27175607. Molecules. 2022. PMID: 36080373 Free PMC article. Review.
References
-
- American Cancer Society. Cancer facts & figures 2009 (2010) http://www.cancer.org/downloads/STT/500809web.pdf. Accessed June 4, 2010
-
- Tsao H, Atkins MB, Sober AJ. Management of cutaneous melanoma. N Engl J Med. 2004;351:998–1012. - PubMed
-
- Robert C, Thomas L, Bondarenko I, O’Day S, Weber J, Garbe C et al (2011) Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. New Engl J Med (June 6 [Epub ahead of print]) - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

