Population pharmacokinetic and exposure-response analysis of nilotinib in patients with newly diagnosed Ph+ chronic myeloid leukemia in chronic phase
- PMID: 22207416
- PMCID: PMC5557053
- DOI: 10.1007/s00228-011-1200-7
Population pharmacokinetic and exposure-response analysis of nilotinib in patients with newly diagnosed Ph+ chronic myeloid leukemia in chronic phase
Abstract
Purpose: We investigated the population pharmacokinetics and exposure-response relationship of nilotinib in patients with newly diagnosed chronic myeloid leukemia (CML) in chronic phase.
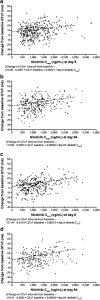
Methods: Nilotinib was given at 300 mg or 400 mg twice daily. Serum concentration data (sparse and full pharmacokinetic profiles) were obtained from 542 patients over 12 months. A population pharmacokinetic analysis was performed using nonlinear mixed-effect modeling. Exposure-response relationships were explored graphically or using logistic regression models.
Results: Nilotinib concentrations were stable over 12 months. Patients in the 400 mg twice-daily arm had an 11.5% higher exposure than did those in the 300 mg twice-daily arm, and the relative bioavailability of nilotinib 400 mg twice daily was 0.84 times that of 300 mg twice daily. Patient demographics did not significantly affect nilotinib pharmacokinetics. The occurrence of all-grade total bilirubin elevation was significantly higher in patients with higher nilotinib exposure, and a positive correlation was also observed between nilotinib exposure and QTcF change on electrocardiograms from baseline. There was no significant relationship between nilotinib exposure and major molecular response at 12 months.
Conclusions: There is a less than proportional dose-exposure relationship between nilotinib 300 mg and 400 mg twice-daily doses. Blood level testing is unlikely to play an important role in the general management of patients with newly diagnosed CML treated with nilotinib.
Trial registration: ClinicalTrials.gov NCT00471497.
Conflict of interest statement
Figures




Comment in
-
Bile acid is important for gastrointestinal absorption of nilotinib.Eur J Clin Pharmacol. 2012 Nov;68(11):1575-6; author reply 1573-4. doi: 10.1007/s00228-012-1282-x. Epub 2012 Apr 15. Eur J Clin Pharmacol. 2012. PMID: 22527347 No abstract available.
References
-
- Tasigna [package insert] East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2010.
-
- Manley PW, Drueckes P, Fendrich G, Furet P, Liebetanz J, Martiny-Baron G, Mestan J, Trappe J, Wartmann M, Fabbro D. Extended kinase profile and properties of the protein kinase inhibitor nilotinib. Biochim Biophys Acta. 2010;1804(3):445–453. - PubMed
-
- Weisberg E, Manley PW, Breitenstein W, Bruggen J, Cowan-Jacob SW, Ray A, Huntly B, Fabbro D, Fendrich G, Hall-Meyers E, Kung AL, Mestan J, Daley GQ, Callahan L, Catley L, Cavazza C, Azam M, Neuberg D, Wright RD, Gilliland DG, Griffin JD. Characterization of AMN107, a selective inhibitor of native and mutant Bcr-Abl. Cancer Cell. 2005;7(2):129–141. - PubMed
-
- Rosti G, Palandri F, Castagnetti F, Breccia M, Levato L, Gugliotta G, Capucci A, Cedrone M, Fava C, Intermesoli T, Cambrin GR, Stagno F, Tiribelli M, Amabile M, Luatti S, Poerio A, Soverini S, Testoni N, Martinelli G, Alimena G, Pane F, Saglio G, Baccarani M GIMEMA CML Working Party. Nilotinib for the frontline treatment of Ph(+) chronic myeloid leukemia. Blood. 2009;114(24):4933–4938. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

