Biogenesis of Weibel-Palade bodies in von Willebrand's disease variants with impaired von Willebrand factor intrachain or interchain disulfide bond formation
- PMID: 22207689
- PMCID: PMC3366651
- DOI: 10.3324/haematol.2011.057216
Biogenesis of Weibel-Palade bodies in von Willebrand's disease variants with impaired von Willebrand factor intrachain or interchain disulfide bond formation
Abstract
Background: Mutations of cysteine residues in von Willebrand factor are known to reduce the storage and secretion of this factor, thus leading to reduced antigen levels. However, one cysteine mutation, p.Cys2773Ser, has been found in patients with type 2A(IID) von Willebrand's disease who have normal plasma levels of von Willebrand factor. We hypothesize that disruption of either intra- or interchain disulfide bonds by cysteine mutations in von Willebrand factor has different effects on the biogenesis of Weibel-Palade bodies.
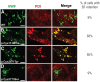
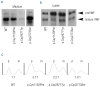
Design and methods: The effect of specific cysteine mutations that either disrupt intrachain (p.Cys1130Phe and p.Cys2671Tyr) or interchain (p.Cys2773Ser) disulfide bonds on storage and secretion of von Willebrand factor was studied by transient transfection of human embryonic kidney cell line 293. Upon expression of von Willebrand factor these cells formed endothelial Weibel-Palade body-like organelles called pseudo-Weibel-Palade bodies. Storage of von Willebrand factor was analyzed with both confocal immunofluorescence and electron microscopy. Regulated secretion of von Willebrand factor was induced by phorbol 12-myristate 13-acetate.
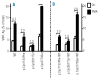
Results: p.Cys1130Phe and p.Cys2671Tyr reduced the storage of von Willebrand factor into pseudo-Weibel-Palade bodies with notable retention of von Willebrand factor in the endoplasmic reticulum, whereas p.Cys2773Ser-von Willebrand factor was stored normally. As expected, wild-type von Willebrand factor formed proteinaceous tubules that were seen under electron microscopy as longitudinal striations in pseudo-Weibel-Palade bodies. p.Cys2773Ser caused severe defects in von Willebrand factor multimerization but the factor formed normal tubules. Furthermore, the basal and regulated secretion of von Willebrand factor was drastically impaired by p.Cys1130Phe and p.Cys2671Tyr, but not by p.Cys2773Ser.
Conclusions: We postulate that natural mutations of cysteines involved in the formation of interchain disulfide bonds do not affect either the storage in Weibel-Palade bodies or secretion of von Willebrand factor, whereas mutations of cysteines forming intrachain disulfide bonds lead to reduced von Willebrand factor storage and secretion because the von Willebrand factor is retained in the endoplasmic reticulum.
Figures



Similar articles
-
Insights into pathological mechanisms of missense mutations in C-terminal domains of von Willebrand factor causing qualitative or quantitative von Willebrand disease.Haematologica. 2013 Aug;98(8):1315-23. doi: 10.3324/haematol.2013.084111. Epub 2013 Mar 28. Haematologica. 2013. PMID: 23539537 Free PMC article.
-
Storage and secretion of naturally occurring von Willebrand factor A domain variants.Br J Haematol. 2014 Nov;167(4):529-40. doi: 10.1111/bjh.13074. Epub 2014 Aug 8. Br J Haematol. 2014. PMID: 25103891
-
Re-establishment of VWF-dependent Weibel-Palade bodies in VWD endothelial cells.Blood. 2005 Jan 1;105(1):145-52. doi: 10.1182/blood-2004-02-0464. Epub 2004 Aug 26. Blood. 2005. PMID: 15331450 Free PMC article.
-
Weibel-Palade bodies: a window to von Willebrand disease.J Thromb Haemost. 2013 Apr;11(4):581-92. doi: 10.1111/jth.12160. J Thromb Haemost. 2013. PMID: 23398618 Review.
-
How to roll an endothelial cigar: the biogenesis of Weibel-Palade bodies.Traffic. 2004 Feb;5(2):69-78. doi: 10.1111/j.1600-0854.2004.00157.x. Traffic. 2004. PMID: 14690496 Review.
Cited by
-
Insights into pathological mechanisms of missense mutations in C-terminal domains of von Willebrand factor causing qualitative or quantitative von Willebrand disease.Haematologica. 2013 Aug;98(8):1315-23. doi: 10.3324/haematol.2013.084111. Epub 2013 Mar 28. Haematologica. 2013. PMID: 23539537 Free PMC article.
-
Characterization of large in-frame von Willebrand factor deletions highlights differing pathogenic mechanisms.Blood Adv. 2020 Jul 14;4(13):2979-2990. doi: 10.1182/bloodadvances.2018027813. Blood Adv. 2020. PMID: 32609846 Free PMC article.
-
Von Willebrand Disease: From In Vivo to In Vitro Disease Models.Hemasphere. 2019 Sep 27;3(5):e297. doi: 10.1097/HS9.0000000000000297. eCollection 2019 Oct. Hemasphere. 2019. PMID: 31942548 Free PMC article. Review.
-
Variability of von Willebrand factor-related parameters in endothelial colony forming cells.J Thromb Haemost. 2019 Sep;17(9):1544-1554. doi: 10.1111/jth.14558. Epub 2019 Jul 22. J Thromb Haemost. 2019. PMID: 31265169 Free PMC article.
-
Sec22b determines Weibel-Palade body length by controlling anterograde ER-Golgi transport.Haematologica. 2021 Apr 1;106(4):1138-1147. doi: 10.3324/haematol.2019.242727. Haematologica. 2021. PMID: 32336681 Free PMC article.
References
-
- Sadler JE. Biochemistry and genetics of von Willebrand factor. Annu Rev Biochem. 1998;67:395–424. - PubMed
-
- Sporn LA, Marder VJ, Wagner DD. Inducible secretion of large, biologically potent von Willebrand factor multimers. Cell. 1986;46(2):185–90. - PubMed
-
- Giblin JP, Hewlett LJ, Hannah MJ. Basal secretion of von Willebrand factor from human endothelial cells. Blood. 2008;112(4):957–64. - PubMed
-
- Wagner DD. Cell biology of von Willebrand factor. Annu Rev Cell Biol. 1990;6:217–46. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

